-
With the rise of e-commerce platforms and rapid development of logistics systems, the transportation chain for raw materials and processed products has been extended, imposing higher requirements for maintaining the quality of meat and meat products during storage and transportation. Existing preservation technologies struggle to meet consumer demands, making the optimization of packaging systems to maintain meat freshness during the storage period a significant challenge faced by the food industry. Active packaging (AP) is a technology that extends food shelf life by incorporating active components to modify the packaging environment[1], and common preparation methods include extrusion, blow molding, casting, and stretching[2,3]. In recent years, electrospinning has gained considerable attention as a novel AP fabrication approach. This technique uses high voltage to transform polymer solutions into continuous fibers with diameters ranging from micrometers to nanometers[4], typically employing a system composed of a high-voltage power supply, syringe pump, spinneret, and collector[5]. Compared to conventional methods, electrospinning offers advantages such as operational simplicity, precise process control, and high flexibility. The resulting fibrous membranes exhibit high porosity, large specific surface area, and excellent encapsulation efficiency, making them an effective strategy for incorporating bioactive compounds into fibers[6,7]. As a hydrolyzed product of collagen, gelatin (GE) possesses abundant sources, high biocompatibility, and strong biodegradability[8], making it an ideal carrier for active ingredients. Various bioactive components such as curcumin and essential oils have been successfully encapsulated within gelatin matrices to develop composite fibrous membranes with antioxidant and antibacterial properties through electrospinning technology[9,10].
The release rate of active components directly determines food preservation efficacy. Insufficient protection or premature release may lead to reduced activity and inadequate delivery duration during storage[11], resulting in suboptimal performance that fails to achieve expected outcomes while increasing economic costs for manufacturers. Consequently, there is an urgent need to develop sustained-release packaging technologies capable of concentrating the release of active substances during storage. Common triggering mechanisms include light, enzymes, water activity, and pH. Among these, light stimulation enables non-contact triggered release, representing an effective activation method[12]. Ultraviolet (UV) light consists of three wavelength bands: UVA (320~400 nm), UVB (280~320 nm), and UVC (200~280 nm). It not only inhibits microbial nucleic acid transcription and disrupts the synthesis of protein and enzyme, ultimately causing microbial death[13], but also stimulates the release of active components from food packaging into the micro-environment inside the package by destroying the matrix structure via free radical reactions, inducing the electron transition of photosensitive substances, and converting light energy into heat energy, etc.[14−16], thus enhancing their functional effects.
Oregano essential oil (OEO), one of the most widely used plant essential oils, exhibits excellent free radical scavenging capacity and antimicrobial activity for food preservation. However, high volatility limits its practical application in the food industry[17]. Emulsion encapsulation is an effective method to enhance the stability and bioavailability of essential oils[18]. A protein-polysaccharide composite emulsifier composed of gelatin and gum arabic (GA) can form a interfacial network at oil-water interfaces to improve emulsion stability and prevent the uncontrolled release of OEO[19]. Zinc oxide nanoparticles (ZnO NPs), known for their low toxicity and strong antibacterial properties[20], can generate abundant free radicals under UV light, which can disrupt the GE-GA network structure on the interface layer, thus introducing the stimulated and controlled release of active ingredients in the food sterilization process synchronously. In this study, the optimal spinning solution was determined by evaluating emulsion stability. Using electrospinning technology, a gelatin-based composite fibrous membrane embedded with ZnO NPs and OEO was successfully fabricated, establishing a UV-stimulated active packaging system. The structural and functional properties of the fibrous membrane before and after UV irradiation were characterized using electronic nose analysis, FT-IR, and antioxidant activity assays. Furthermore, the membrane was applied to preserve chilled chicken breast during storage, aiming to provide insights into meat quality maintenance throughout storage periods.
-
Chilled chicken breast, and PE cling film were bought from Suguo supermarket, Nanjing, Jiangsu Province; B-type gelatin, oregano essential oil, gum arabic, 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) were purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China); Tween-80 was purchased from Anage Chemical; Zinc oxide nanoparticles (ZnO NPs), 2,2-diazo-bis (3-ethyl-benzothiazole-6-sulfonic acid) diamiammonium salt (ABTS) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China).
Preparation and optimization of spinning solutions
-
The preparation of essential oil emulsion was adapted from the methods of Zhang et al.[19,21] with some modifications. First, gelatin and gum arabic were dissolved separately in 40% (v/v) acetic acid solution and ultrapure water, respectively. The solutions were mixed at a mass ratio of 2:1 to form gelatin-gum arabic solutions at different concentrations (5%, 10%, 15%, 20%, and 25% w/v), and the pH was adjusted to 3.6 with 2M NaOH solution. The solutions were then left at 25 °C for 24 h. Subsequently, 2% (w/v) zinc oxide nanoparticles and 20% (w/v) oregano essential oil were slowly added sequentially to the solution, and homogenized at 12000 rpm for 4 min. After homogenization, the essential oil emulsions were obtained and labeled as EA5, EA10, EA15, EA20, and EA25, respectively. The prepared emulsions were allowed to stand for a period at room temperature to observe their stratification and assess emulsion stability. The optimal group was selected for subsequent sample preparation.
A 20% (w/v) gelatin (GE) solution was prepared by dissolving gelatin in acetic acid solution. Then, the GE solution was mixed with the selected optimal emulsion at a 1:1 volume ratio, followed by the addition of 0.5% (w/v) Tween-80 and thorough mixing to obtain the final spinning solution, designated as GE-EA.
Preparation of composite fibrous membranes and selection of spinning parameters
-
The prepared spinning solution was loaded into a 10 mL syringe for electrospinning (JDF05 electrostatic spinning machine, Nano Instrument Technology Co., Changsha, China), and the solidified fibers were collected on oil-coated paper attached to a roller. The total spinning solution volume was set to 4.5 mL, with a 21 G needle, a spinning voltage of 16~20 kV, a solution feed rate of 0.15~0.35 mL/h, and a receiving distance of 17.5 cm. The spinning temperature was maintained at 25°C, and the ambient relative humidity was controlled below 50%. A glass slide was used as a receiver to collect a portion of the spun product, which was then placed under a fluorescent upright microscope (Axio Scope A1 Fluorescent upright microscope, ZEISS, Germany) for observation, with the objective lens magnification adjusted to 10×.
Structural characterization of composite fibrous membranes
-
The film samples were analyzed using a Fourier transform infrared (FT-IR) spectrometer (Antaris II, Thermo Fisher Scientific, USA), following the method of Shen et al.[22] with some modifications. Before measurement, the spun fibrous membranes were irradiated with a UVC lamp (Philips Lighting Co., Ltd, Netherlands) for 0 and 4 h. The scanning range was 4,000~500 cm−1, with a resolution of 40 cm−1, and the tests were conducted at room temperature.
Electronic nose analysis of composite fibrous membranes
-
The prepared composite fibrous membranes were cut into 2 cm × 2 cm squares and placed in headspace vials. After sealing, the samples were irradiated with a UVC lamp for 0 and 4 h. After treatment, the vials were placed in a 50 °C water bath every 5 min, heated for 30 min, and then subjected to electronic nose analysis (PEN3, Airsense, Germany). For comparison, 2 mL of oregano essential oil was also analyzed using the same method to evaluate flavor compounds. Table 1 shows the corresponding substance response types for each sensor of the electronic nose.
Table 1. Performance description of electronic nose sensors.
Array ID Sensor name Sensitive components R1 W1C Aromatic compounds R2 W5S Nitrogen oxides R3 W3C Ammonia compounds R4 W6S Selective to hydrides R5 W5C Short-chain alkanes and aromatic compounds R6 W1S Methyl compounds R7 W1W Inorganic sulfides R8 W2S Alcohols, aldehydes and ketones R9 W2W Aromatic compounds and organic sulfides R10 W3S Long-chain alkanes Dynamic antioxidant properties of composite fibrous membranes
-
The ABTS radical scavenging activity was determined according to the method of Xu et al.[23] with slight modifications. ABTS solution and potassium persulfate solution were prepared separately and mixed at a 1:1 ratio to obtain an ABTS+ stock solution. The mixture was kept in the dark at room temperature for a certain period. Subsequently, the stock solution was diluted with 0.2 M phosphate-buffered saline (PBS, pH 7.4) until the absorbance at 734 nm reached 0.7 ± 0.02 for subsequent testing. A 2 cm × 2 cm film sample was placed in a test tube and irradiated with a UVC lamp for 0 and 4 h. Then, 4 mL of the reaction solution was added to the tube, followed by dark incubation for different durations. The absorbance of the sample at 734 nm was measured. The ABTS radical scavenging capacity of the composite fibrous membranes was calculated using Eqn (1):
$ {\text{ABTS scavenging activity}}\; ({\text{%}}) = \dfrac{{{A_{{0}}} - {A_1}}}{{{A_0}}} \times 100 $ (1) where, A0 is the absorbance of the ABTS blank solution, and A1 is the absorbance of the reaction solution.
The DPPH radical scavenging activity was measured according to the method of Xu et al.[23] with slight modifications. A 2 cm × 2 cm film sample was placed in a test tube, and after UV irradiation for 0 and 4 h, 4 mL of 0.2 mM DPPH solution (dissolved in methanol) was added to each tube. The tubes were vortexed for 1 min and then incubated in the dark for different durations. The absorbance of the reaction solution was measured at 517 nm. The DPPH radical scavenging capacity of the electrospun films was calculated using Eqn (2):
$ {\text{DPPH scavenging activity }}({\text{%}}) = \dfrac{{{A_{\text{0}}} - {A_1}}}{{{A_0}}} \times 100 $ (2) where, A0 is the absorbance of the DPPH blank solution, and A1 is the absorbance of the reaction solution.
Preservation application of composite fibrous membranes in chilled chicken breast
-
The prepared composite fibrous membranes and commercially available PE cling film were used to package chilled chicken breast using air packaging. Care was taken to ensure no direct contact between the fibrous membrane and the chicken breast. The packaged samples were stored at 4 °C, and the total bacterial count of the chilled meat was measured on day 0 and day 6, following the operational method specified in GB 4789.2-2022. Briefly, 10 g of the sample was mixed with 90 mL of saline solution, homogenized by stomaching, and then 0.5 mL of the initial solution was aspirated and added to 4.5 mL of sterile saline. After gradient dilution to an appropriate concentration, 100 μL was taken for plate spreading, followed by incubation at 37 °C for 24~48 h before colony counting.
Statistical analysis
-
All experiments were performed in triplicate. Data were preliminarily organized using Microsoft Office 2021, and graphs were generated using Origin 2024 software. One-way ANOVA was performed on the data using IBM SPSS Statistics 26 software. Results were expressed as mean ± standard deviation, and statistical significance was set at p < 0.05.
-
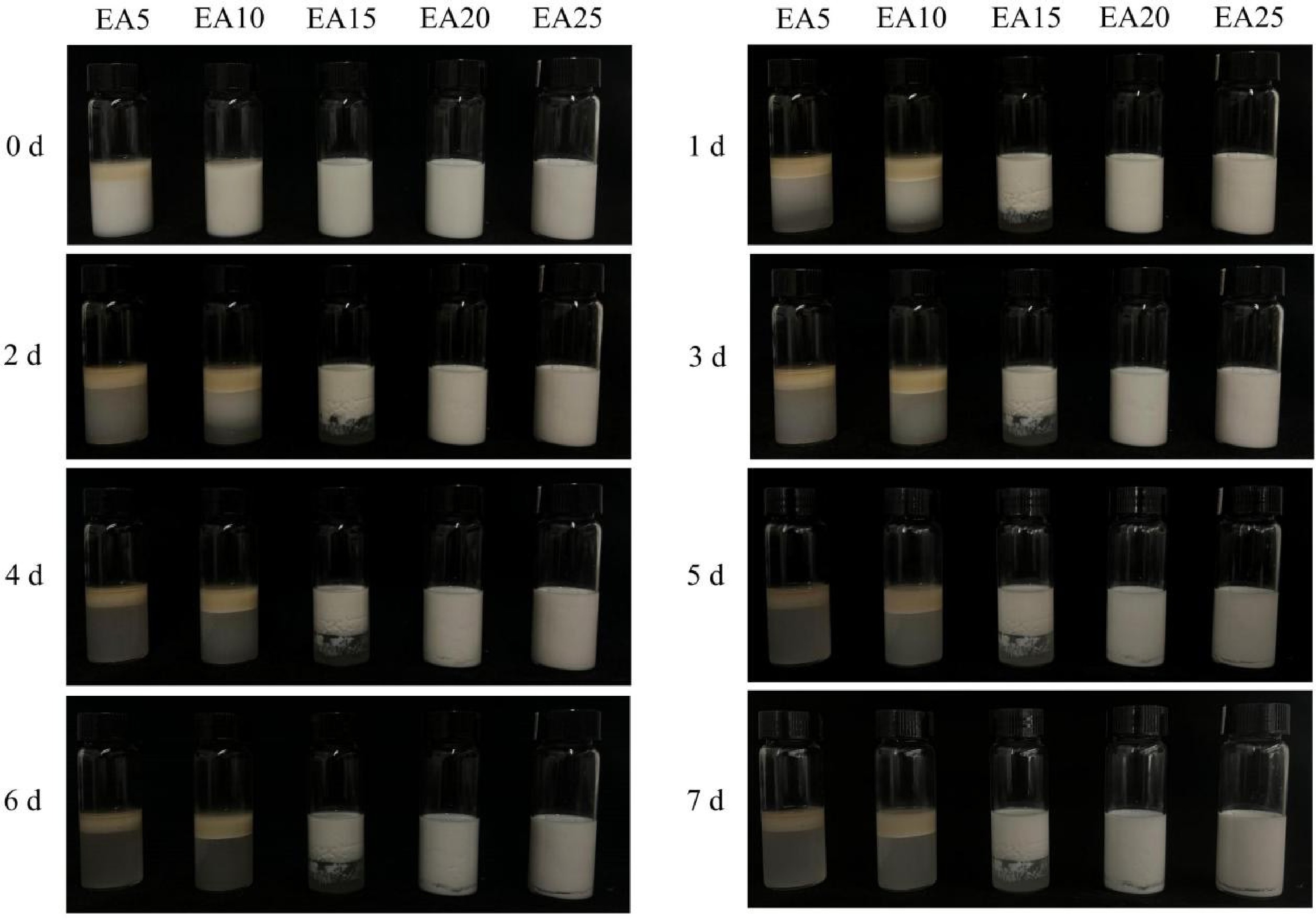
Enhancing the stability of essential oil emulsions is a prerequisite for reducing the ineffective release of OEO during fibrous membrane preparation and storage. Emulsifiers, with their amphiphilic nature, can adsorb at the oil-water interface to form an interfacial layer, playing a crucial role in improving emulsion stability[24]. A gelatin-gum arabic mixed solution can form a protein-polysaccharide complex and interact through hydrogen bonding and electrostatic attraction to create a composite interfacial layer under appropriate pH conditions, significantly enhancing emulsion stability[19]. Previous studies have shown that gelatin first adsorbs at the oil-water interface to reduce interfacial tension, while gum arabic further improves stability by increasing the viscosity of the continuous phase[25,26]. OEO emulsions were prepared using mixed solutions of gelatin and gum arabic at different concentrations and were left to stand at room temperature (Fig. 1). When the concentrations of gelatin-gum arabic solutions were 5% and 10% (w/v), phase separation occurred within one day of storage, with the less dense OEO separating from the emulsion and floating to the top. Notably, the EA5 group began to separate shortly after preparation. At a 15% (w/v) gelatin-gum arabic concentration, the OEO emulsion exhibited coalescence and flocculation after one day of storage. However, when the concentration of the gelatin-gum arabic solution was increased to 20% (w/v) or higher, the storage stability of the essential oil emulsion significantly improved, with no phase separation observed within the first 3 d. The EA20 and EA25 treatment groups began to exhibit slight flocculation only on the 4th and 5th d of storage respectively, with no significant difference observed between the two groups. This enhancement is likely due to the thickened interfacial layer and increased emulsion viscosity, which hinder droplet aggregation and upward movement of oil droplets[21,27]. Therefore, the EA20 and EA25 emulsions were selected for subsequent preparation of spinning solutions and fibrous membranes.
The screening of electrospinning conditions
-
During the electrospinning process, the spinning solution is extruded from the spinneret at a constant speed by a propulsion pump. Upon applying voltage, the polymer droplet is stretched, forming a 'Taylor cone' at the needle tip under the combined action of electric field force and surface tension[28]. As the electric field force further increases, the charged droplet overcomes surface tension and is released from the tip of the Taylor cone, forming a jet that propels toward the collector. During flight, the solvent evaporates, and the jet undergoes further splitting and thinning due to electrostatic repulsion and other interactive forces, ultimately solidifying on the collector to form a nanofiber membrane[29]. Numerous factors influence electrospinning, including the properties of the spinning solution, environmental conditions, and process parameters such as collection distance, voltage, solution flow rate, and needle size[30]. This study conducted a comparative investigation of these complex process parameters, optimizing and screening the best conditions for preparing electrospun membranes (Table 2).
Table 2. Electrospinning state under different conditions.
Spinning solution Feed rate (mL/h) Spinning voltage (kV) Spinning state EA20 0.15~0.35 16~20 Dense jet flow but ejected material was powdery EA25 0.15~0.35 16~20 Dense jet flow but ejected material was powdery GE-EA20 0.15~0.35 16~20 Inadequate fiber stretching at nozzle, droplet spraying occured, with powdery output GE-EA25 0.15 16 Inadequate fiber stretching at nozzle 18 Insufficient stretching velocity resulted in non-continuous spinning 20 Insufficient stretching velocity resulted in non-continuous spinning 0.35 16 Insufficient nozzle stretching led to droplet spraying 18 Continuous spinning was achieved with stable fiber formation 20 Continuous spinning was realized with fiber formation, yet the jet stream remained unstable Electrospinning was performed using pure emulsions EA20 and EA25, as well as the GE-EA20 group with an added 20% (w/v) gelatin solution. It was found that the collected material on the oil paper appeared as powder rather than fibrous filaments. This may be attributed to the low polymer molecular weight and insufficient solution viscosity, causing the spinning jet to favor electrostatic spraying. Additionally, the surface tension of GE-EA20 could not balance the electric field force, making it difficult for the electric field to overcome surface tension and form a stable Taylor cone for sufficient droplet stretching[30]. In contrast, due to the increased gelatin-gum arabic concentration in EA25, which helped reduce surface tension, the GE-EA25 group successfully produced fibrous filaments during electrospinning. At a flow rate of 0.15 mL/h, the slow solution feed rate prevented continuous jet formation; increasing the flow rate to 0.35 mL/h enabled continuous spinning, with optimal performance achieved at 18 kV, yielding fine-diameter fibers under microscopic observation, as is shown in Fig. 2. However, when the voltage was further increased to 20 kV, excessive electric field strength caused localized discharge and heating, leading to rapid solvent evaporation and unstable jet formation[31]. Similarly, Topuz & Uyar[32] electrospun a 25% (w/v) gelatin solution and observed that increasing the voltage from 10 to 20 kV transformed the fibers from well-defined circular morphologies into undesirable flattened shapes. Dai et al.[33] successfully obtained uniform nanofibers by electrospinning sodium dodecyl sulfate-gelatin-stabilized corn oil emulsions at a feed rate of 0.3 mL/h and an applied voltage of 17~21 kV. Based on these findings, this study selected the GE-EA25 spinning solution, a feed rate of 0.35 mL/h, and a spinning voltage of 18 kV for the preparation of composite fibrous membranes.
Structural characterization of composite fibrous membranes
-
As shown in Fig. 3, the effect of UV irradiation on the FTIR spectra of the composite fibrous membranes was investigated. The results revealed a broad absorption peak at around 3,280 cm−1, attributed to the stretching vibrations of hydroxyl and amino groups in gelatin and oregano essential oil[34]. After 4 h of UVC irradiation, this broad peak slightly shifted toward higher wavenumbers, indicating that UV irradiation disrupted the hydrogen bonding structure. The absorption band near 2,945 cm−1 corresponds to the asymmetric stretching vibration of methylene groups, confirming the successful incorporation of oregano essential oil into the composite fibrous membrane, a similar phenomenon was observed in the study by Zhang et al.[35]. After UV irradiation, the intensity of this peak increased, and its shape became sharper, suggesting that UV light stimulated the release of oregano essential oil from the fiber matrix and its migration to the surface[36]. The peaks at approximately 1,636 and 1,545 cm−1 were assigned to the C=O stretching vibration of amide I and the C-N bending/stretching vibration of amide II, respectively[37]. Under UV treatment, the interaction mode between ZnO NPs and C=O may have altered. The conjugation effect reduced the electron cloud density around the oxygen atom in C=O, decreasing the bond force constant and causing a redshift in the vibration frequency. Additionally, the weakened peak intensity at 1,636 cm−1 after UV irradiation indicated a reduction in intermolecular interactions within the fibrous membrane. Furthermore, the symmetric stretching vibration peak of -COO− shifted from 1,401 to 1,397 cm−1 with a notable decrease in intensity, likely due to the generation of free radicals by ZnO NPs under UV irradiation, leading to the cleavage of molecular chains in the fiber matrix[23]. The characteristic peak near 1,030 cm−1 may represent the asymmetric C-O-C stretching of Tween-80[38], which exhibited a slight redshift after UV treatment. The FTIR results demonstrated good miscibility among oregano essential oil, ZnO NPs, and the fibrous membrane. Moreover, UV irradiation disrupted the film structure by weakening intermolecular forces, facilitating the release of essential oil and enhancing its bioactive performance.
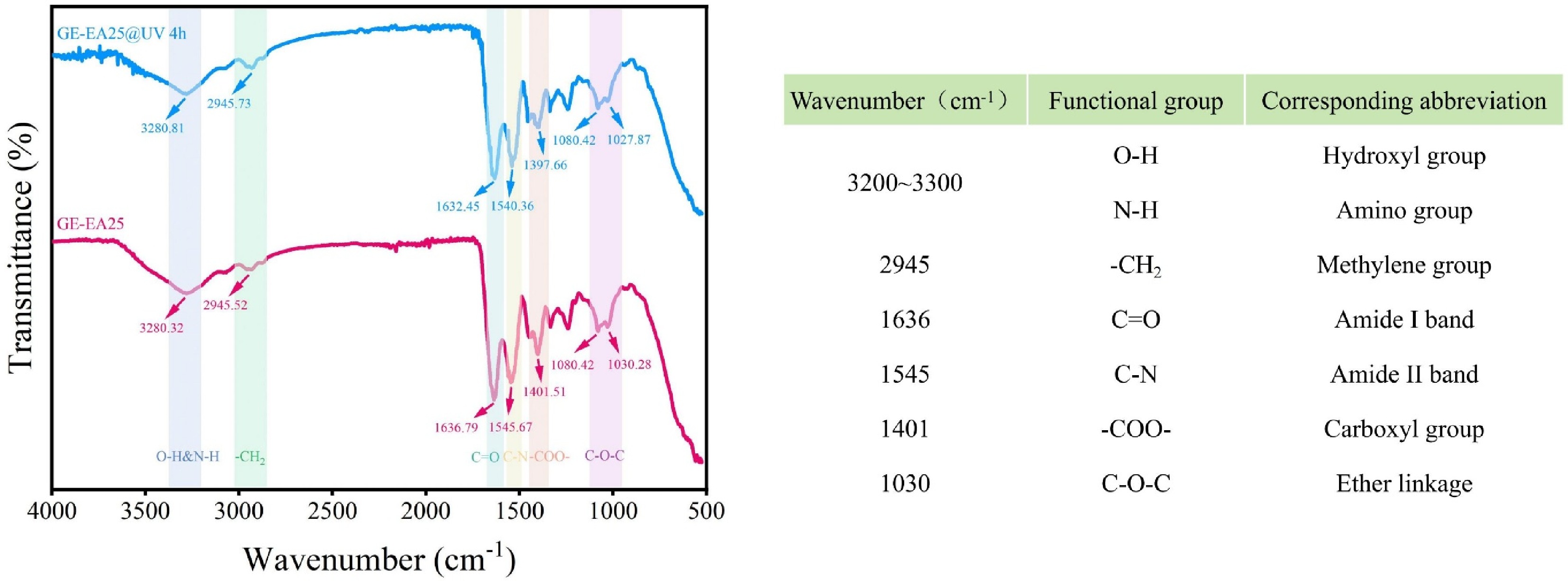
Figure 3.
Effect of UV irradiation on FT-IR of fibrous membranes (GE-EA25: Fiber film prepared with 20% (w/v) gelatin solution as the film-forming matrix and 25% (w/v) concentration of gelatin-gum arabic composite emulsifier; GE-EA25@UV 4 h: GE-EA25 composite fibrous membrane irradiated by UVC for 4 h).
Electronic nose analysis of composite fibrous membranes
-
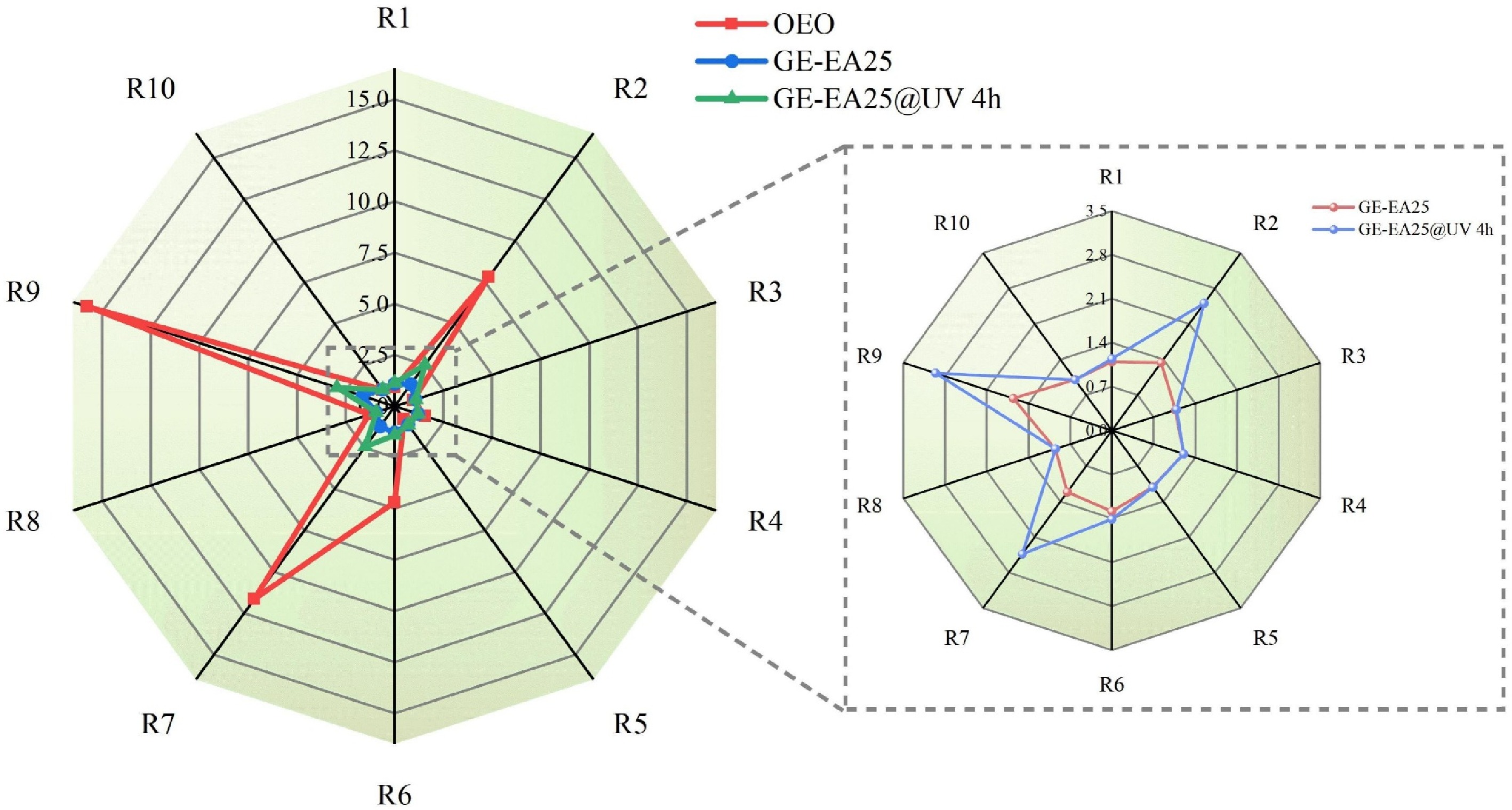
The electronic nose (e-nose) was employed to detect volatile components in the samples. The substances that exert antioxidant, antibacterial, and other active effects in plant essential oils are mainly the volatile flavor components, such as terpenoids, alcohols, and phenols. The radar plot of e-nose signals for oregano essential oil is presented in Fig. 4. The results indicated that the aroma profile was predominantly detected by four sensors: R2 (sensitive to nitrogen oxides), R6 (sensitive to methyl compounds), R7 (responsive to inorganic sulfides), and R9 (specialized for aromatic compounds and organic sulfides). Compared to the non-irradiated GE-EA25 group, the UV-stimulated fibrous membrane (GE-EA25@UV 4 h) exhibited increased sensor responses, with R2, R7, and R9 showing the most significant enhancement, which were substantially higher than those of the untreated group and were consistent with the radar pattern of pure oregano essential oil. The e-nose data demonstrate that UVC irradiation promotes the release of volatile flavor compounds such as carvacrol and thymol from the fibrous membrane, confirming its ability to stimulate the release of essential oil.
Dynamic antioxidant properties of composite fibrous membranes
-
Figure 5a illustrates the effect of UV irradiation on the dynamic DPPH radical scavenging activity of electrospun fibrous membranes. After dark incubation for 0.67, 3, 6, 24, 48, 72, and 96 h, both membrane groups exhibited an overall increasing trend in DPPH scavenging rate over time. The enhancement in scavenging efficiency can be attributed to two concurrent mechanisms: firstly, the prolonged interaction between essential oils and free radicals over extended reaction time; secondly, the progressive increase in essential oil release from the composite fiber matrix throughout the reaction duration[23]. After 3 d of reaction, the UV-stimulated GE-EA25@UV 4 h group demonstrated significantly higher DPPH scavenging capacity than the non-irradiated group (p < 0.05). This is because gelatin-gum arabic and ZnO NPs, as emulsifiers, encapsulate oregano essential oil in emulsion form. Notably, ZnO NPs are photosensitive and can generate ROS under UV irradiation[20], which disrupt protein and polysaccharide structures, leading to the disintegration of the emulsion interface. This created additional release pathways for the essential oil, increasing free bioactive compounds in the reaction system and thereby boosting antioxidant performance.
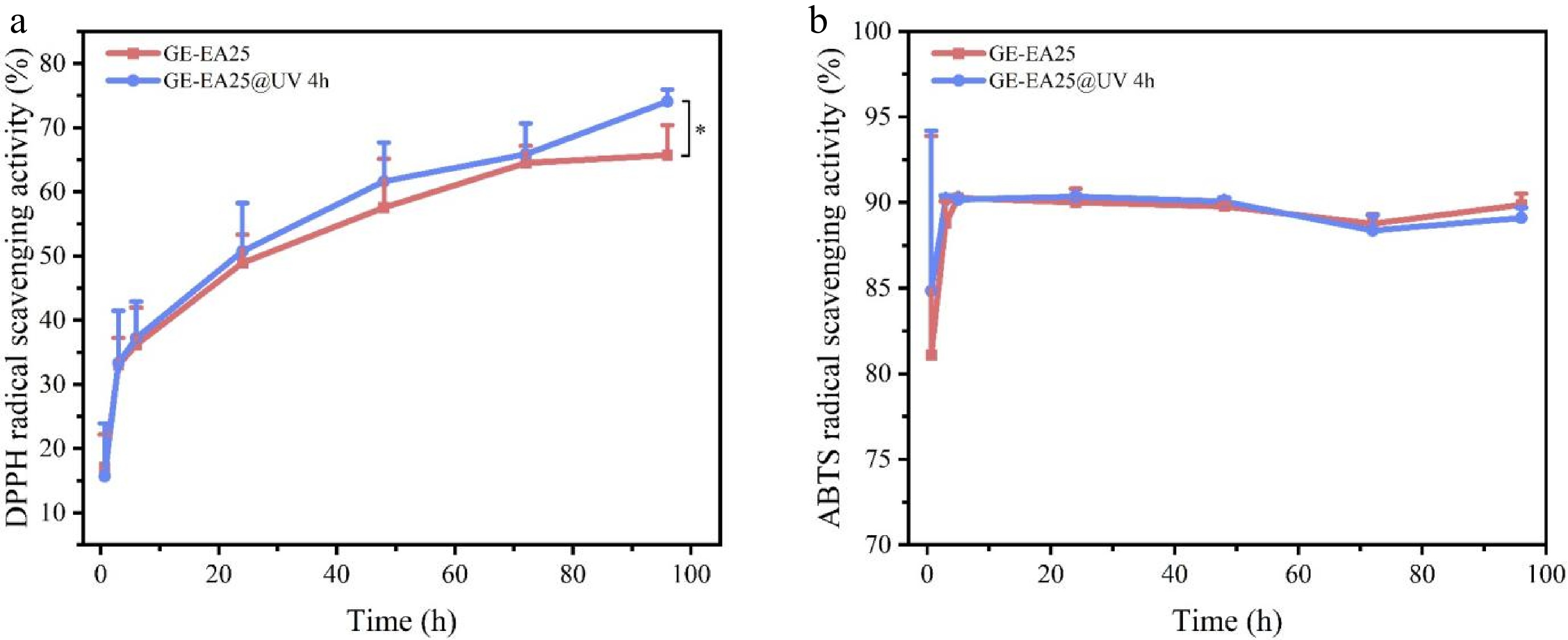
Figure 5.
Effect of UV irradiation on antioxidant properties of fibrous membranes. (a) DPPH; (b) ABTS.
Figure 5b shows the dynamic ABTS radical scavenging activity of the composite membranes. Initially (0 h), both groups exhibited over 80% ABTS scavenging rates, which slightly increased over time and stabilized around 89%, and the GE-EA25@UV 4 h group maintained relatively stable activity. This behavior likely stems from the water-soluble gelatin matrix of the fibrous membranes. Upon contact with the ABTS working solution, the film rapidly dissolved, releasing the essential oil directly into the reaction medium and achieving saturation at the initial stage.
In summary, the dynamic antioxidant assessment demonstrates that the prepared electrospun fibrous membranes exhibit both high antioxidant capacity and sustained-release properties, with UV light serving as an effective trigger for controlled release, which corroborates with the electronic nose analysis results.
Preservation application of composite fibrous membranes in chilled chicken breast
-
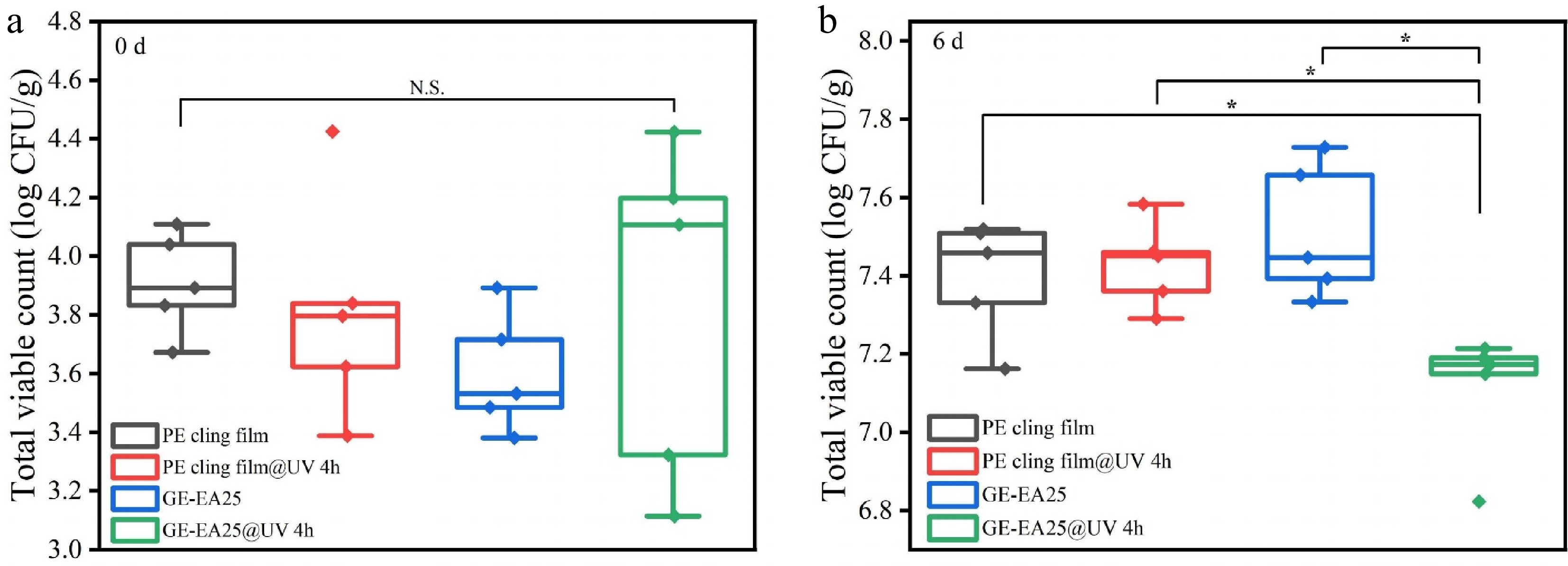
Total viable count (TVC) serves as a crucial indicator for evaluating food freshness. In this experiment, the prepared fibrous membranes were used for air packaging of chilled chicken breast, with commercial PE cling film as control. TVC was measured on day 0 and day 6, and Fig. 6 shows the TVC changes in chilled chicken breast under different packaging conditions. On day 0, all samples showed TVC below 4 log CFU/g (3.91, 3.81, 3.61, and 3.83 log CFU/g, respectively), indicating consistent initial microbial loads that met hygiene and quality requirements. During storage, TVC increased significantly (p < 0.05). By day 6, all groups exceeded 7 log CFU/g (7.39, 7.43, 7.51, and 7.11 log CFU/g, respectively).
The non-UV-stimulated GE-EA25 group showed the highest TVC, though not significantly different from the control group (p > 0.05). This may be attributed to the electrospun fibrous membrane's large surface area, high porosity, and strong breathability compared to the dense structure of cling film, creating more pathways for water vapor and oxygen transmission that potentially promoted microbial growth[39]; furthermore, the cling film's high UV transmittance reduced initial TVC through UV irradiation[23], whereas the non-irradiated GE-EA25 group had limited essential oil release from the fiber matrix.
The GE-EA25@UV 4 h group demonstrated significantly slower TVC growth (p < 0.05), achieving the lowest final count despite higher initial loads. This confirms that UV activation promotes oregano essential oil release from the membrane. The phenolic and terpenoid compounds can disrupt microbial cell membranes and enzyme systems[40], inhibiting growth and spore germination, consistent with previous findings. Mith et al.[41] also verified oregano essential oil's antibacterial effects through Shiga toxin reduction via ler gene transcription inhibition in E. coli. In conclusion, UV irradiation reduces initial microbial loads, while the electrospun fibrous membrane synergized with UV excitation facilitates the controlled release of volatile antimicrobial compounds into the package headspace, achieving antibacterial effects without direct food contact.
-
This study initially employed gelatin-gum arabic as a composite emulsifier combined with zinc oxide nanoparticles to encapsulate oregano essential oil. Through evaluation of emulsion stability, 20% and 25% (w/v) gelatin-gum arabic solutions were selected as optimal emulsification conditions for preparing film-forming solutions. Subsequently, UV-stimulated gelatin-based composite fibrous membranes were successfully fabricated via electrospinning technology, with their structural and functional characteristics being characterized before and after UV irradiation. FT-IR and e-nose analysis demonstrated that 4-h UVC irradiation could reduce intermolecular interactions while the free radicals generated by ZnO NPs disrupted the emulsion interfacial structure, thereby promoting the release of oregano essential oil. Dynamic antioxidant assays confirmed the membranes' high antioxidant capacity and sustained-release properties, exhibiting ABTS and DPPH radical scavenging rates exceeding 89% and 65% respectively, with UV treatment further enhancing DPPH scavenging capability. When applied in air packaging of chilled chicken breast coupled with UV treatment, the electrospun fibrous membranes demonstrated significant antibacterial and preservation effects during 4 °C storage. These findings provide valuable references for developing UV-stimulated sustained-release electrospun fibrous membranes, offering practical applications for incorporating and releasing active components in food sterilization processes to maintain product quality. However, future research should further investigate the release mechanism by exploring the UV-stimulated rate-regulating release behavior at the interface layer through release kinetics. Additionally, the antibacterial mechanism should be elucidated in greater depth to establish a theoretical foundation for the UV-stimulated active packaging system's stimulate-release functionality and its fresh-keeping applications in chilled meat preservation.
This work was supported by the National Key R&D Program of China during the 14th Five-Year Plan Period (Grant No. 2024YFD2100101), and the China Agriculture Research System of MOF and MARA (Grant No. CARS-41).
-
The authors confirm contribution to the paper as follows: study conception and design, supervision, project administration, writing-review and editing: Zhao X, Xu X; data collection, formal analysis, draft manuscript preparation: Mao Y; funding acquisition: Xu X. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Mao Y, Zhao X, Xu XL. 2025. Fabrication and application of UV-stimulated sustained-release composite fibrous membrane by electrospinning technology. Food Materials Research 5: e008 doi: 10.48130/fmr-0025-0007
Fabrication and application of UV-stimulated sustained-release composite fibrous membrane by electrospinning technology
- Received: 19 April 2025
- Revised: 27 May 2025
- Accepted: 27 May 2025
- Published online: 25 June 2025
Abstract: As an emerging technology, electrospinning can effectively encapsulate active compounds for active packaging. Notably, stimulating the release of active ingredients by photostimulation is conducive to improving their bioavailability during food packaging and accurately playing the role of preservation. This study prepared an oregano essential oil emulsion using gelatin-gum arabic as a compound emulsifier with zinc oxide nanoparticles (ZnO NPs). The emulsion stability test identified 20% and 25% (w/v) gelatin-gum arabic solutions as optimal for electrospinning. On this basis, gelatin served as the film-forming substrate, incorporating the emulsion to produce composite fibrous membranes via electrospinning. The membranes were then exposed to ultraviolet C (UVC) radiation for 0 and 4 h. Results revealed that UV irradiation activated ZnO NPs, generating reactive oxygen species that disrupted the emulsion interface and fiber structure, facilitating essential oil release. Additionally, UV treatment enhanced the membrane's antioxidant capacity, with DPPH radical scavenging showing sustained release over 4 d. Applied to chilled chicken breast packaging, the UV-stimulated membrane significantly reduced microbial counts by day 6 at 4°C, demonstrating the potential for chilled meat preservation. These findings offer insights for developing UV-stimulated active packaging systems.
-
Key words:
- Electrospinning /
- Fibrous membrane /
- Oregano essential oil /
- UV-stimulated /
- Active packaging