-
Perilla frutescens (L.) Britt., an annual herb of the Lamiaceae family, is widely cultivated in China for its medicinal and culinary uses[1]. According to the Pharmacopoeia of the People's Republic of China (2020 edition), Perilla leaves, stems, and fruits are utilized in traditional medicine for their therapeutic properties[2]. The plant is also valued as a food ingredient and a source of essential oils, with significant global demand[3,4].
In China, the domestication of wild P. frutescens began as early as the Song Dynasty (960–1279 AD), and by the Ming Dynasty (1368–1644 AD), a relatively well-established cultivation system had developed[5,6]. P. frutescens thrives in warm and humid climates, exhibiting strong environmental adaptability. It is now widely cultivated in large-scale plantations across regions such as Jilin, Hebei, Anhui, and Zhejiang Provinces in China[7−10].
In recent years, severe outbreaks of rust disease have been reported in major Perilla-producing regions of China, leading to leaf withering and substantial economic losses[11]. While Coleosporium plectranthi has been identified as the causing agent of Perilla rust in Korea[12], conflicting reports suggest C. perillae as the pathogen in China[13]. This study aimed to resolve this taxonomic ambiguity through morphological and molecular analyses. However, it remains unclear whether the rust pathogen infecting P. frutescens in China is C. plectranthi, C. perillae, or both. To address this question, Perilla rust samples were collected from Beijing and WanNing, Hainan Province, China. The pathogen was identified through morphological characteristics and molecular analyses. These findings provide a theoretical basis for clarifying the taxonomic status of the pathogenic fungus of Perilla rust in China.
Hyperparasitism refers to a biological relationship in which one parasitic organism exploits another parasite as its host, forming a 'parasite-within-a-parasite' interaction. As early as the 1980s, Chinese researchers began studying hyperparasitic fungi associated with pine brown rust (Coleosporium spp.)[14], and some scholars proposed utilizing hyperparasites for plant disease control[15]. Subsequently, hyperparasitism of plant pathogens such as Coniothyrium minitans and Ramularia coleosporii[16] was reported. During field investigations of Perilla rust, it was observed that uredinial pustules of the rust fungus often turned white. This phenomenon closely resembled previous descriptions of Coleosporium zanthoxyli[17] and C. plumeriae[18] hyperparasitized by R. coleosporii. It was therefore hypothesized that the Perilla rust might also be colonized by a hyperparasite. Additionally, it has been reported that the hyperparasite Ramularia coleosporii can cause leaf spot diseases on Campanula rapunculoides, Clematis gouriana, Ipomoea batatas, P. frutescens var. acuta, and P. frutescens[18]. This raises a critical question: Is the white fungus observed a hyperparasite of Perilla rust or a pathogen responsible for Perilla leaf spot? Therefore, the aims of this study were: (i) to identify the pathogen causing Perilla rust in China; (ii) to clarify the taxonomy of the white hyperparasite fungus; (iii) to determine the pathogenicity of the hyperparasite on Perilla leaves; and (iv) to elucidate the hyperparasitism relationship between white fungus and perilla rust fungus. These results will provide essential theoretical insights into the taxonomic status, pathogenicity, and its hyperparasitic interaction with the Perilla rust fungus.
-
Urediniospores and white fungus were collected from infected leaves of 1-year P. frutescens growing in the experimental fields located at the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Beijing (40.04° N, 116.28° E) and Southern Medicinal Botanical Garden of Hainan Branch of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Wanning City, Hainan Province (18.44° N, 110.12° E) with a daily average temperature is 25–30 °C, and a relative humidity of 80% to 95%.
During the present investigation in Hainan and Beijing, nearly all Perilla plants exhibited rust symptom. Notably, the white fungus was present on approximately 10%–20% of the rust-infected plants. For systematic analysis, ten leaf samples from each site were randomly collected, following a randomized sampling protocol. Specifically, each leaf was sampled from a different Perilla plant to ensure representative data collection.
Survey of symptoms in the field
-
During the outbreak of Perilla rust, the infection sites, lesion morphology, and spatial distribution patterns on leaves of P. frutescens were observed. Concurrently, the morphological characteristics of the hyperparasite and its colonization features on uredinial pustules of the Perilla rust fungus were examined.
Isolation and culturing of the hyperparasite
-
The hyphae of the associated hyperparasites from the rust pustules were aseptically transferred to potato dextrose agar (PDA) plates and incubated at 25 °C under a 16-h light/8-h dark cycle. Then, hyphal tips were transferred from the colony margins to a new PDA plate.
Morphological analysis
-
Uredinial pustules of Perilla rust and the conidia of the Perilla rust hyperparasite were transferred onto a glass slide with a drop of sterile water and covered with a coverslip. The morphological characteristics of the urediniospore and the sporulation structures and morphological characteristics of conidia of the hyperparasite were observed under an optical microscope.
Molecular identification of Perilla rust and the hyperparasite fungus
-
Urediniospores from Perilla rust and mycelia of the rust hyperparasitic strain were separately collected, placed into 2 mL microcentrifuge tubes containing steel beads, and ground at 35 Hz for 60 s under liquid nitrogen cooling conditions. Genomic DNA of Perilla rust fungus and its hyperparasite were extracted using the CTAB method[19]. Following the method described by Jia et al.[20], 28S large ribosomal subunit (LSU) rDNA gene fragment of the Perilla rust fungus was amplified using primers LRUST1R (5'-TAAGACCTCAAATCAGGT-3') and LRUST3 (5'-GGGTCATTTAAAGCTAT-3'). The internal transcribed spacer (ITS) region of the rust hyperparasite was amplified using universal primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3').
The PCR amplification system was performed in a total volume of 25 μL, containing: 0.5 μL Taq DNA polymerase (5 U·μL−1), 2.5 μL 10 × PCR buffer (100 mmol·L−1 Tris, 500 mmol·L−1 KCl, 2 mmol·L−1 Mg2+), 2 μL dNTPs (2.5 mmol·L−1), 0.5 μL each of forward and reverse primers (10 µmol·L−1), 1 μL template DNA (~600 ng·μL−1), 18 μL ddH2O. PCR amplification was performed with a initial denaturation at 95 °C for 10 min; followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 51 °C (for Perilla rust fungus)/53 °C (for hyperparasite) for 45 s, and extension at 72 °C for 2 min; with a final extension at 72 °C for 10 min. The amplified products were electrophoresed on a 1% agarose gel and subsequently sent to Sangon Biotech (Shanghai) Co., Ltd. for sequencing. The obtained sequences were subjected to BLAST analysis against homologous sequences in GenBank, and strains with high nucleotide sequence identity were selected. Referring to Yun et al.[12] and Sun et al.[21], phylogenetic trees of Perilla rust fungus and the hyperparasite were constructed using the maximum likelihood (ML) method in MEGA X software, with bootstrap support values calculated from 1,000 replicate samplings.
Determining the taxonomy of Perilla rust and the hyperparasite fungus
-
Based on the microscopic morphological characteristics and molecular sequence alignment results, the taxonomic status of the Perilla rust fungus and the white strain was ultimately determined.
Validation of the hyperparasitic relationship
-
To confirm the hyperparasitic relationship between the white fungus and Perilla rust fungus, three treatments were set: (1) Perilla rust seedlings inoculated with white fungal isolate; (2) Perilla rust seedlings inoculated with sterile PDA plugs; and (3) healthy Perilla seedlings inoculated with white fungal isolate. Perilla rust seedlings were infected naturally, a white fungal isolate was pre-cultured, and three 6-mm-diameter mycelial plugs were placed on each separate leaf per seedling. All seedlings were secured with plastic clips and incubated separately under controlled conditions, with regular misting to maintain relative humidity ≥ 90%. Changes in rust pustules and healthy leaves were observed 7 d post-inoculation (dpi).
-
In total, 2.4 hectares of commercial Perilla fields distributed across five sites (two from Beijing, and three from Hainan) were surveyed, the incidence of Perilla rust was 74.85% ± 5.25%, and the incidence of hyperparasitism was 29.70% ± 2.37%. During the early stage of Perilla rust infection, numerous small, pale yellow chlorotic spots appear on the adaxial (upper) leaf surface (Fig. 1a), while corresponding abaxial (lower) surfaces develop tiny, golden-yellow, circular powdery elevations (uredinial pustules of the rust fungus) (Fig. 1b). As the disease progresses, the number of uredinial pustules increases, and mature pustules gradually turn yellowish-brown (Fig. 1c). Later, infected leaves exhibit withering, curling symptoms, and eventual wilting before abscission. Perilla rust pustules turned white when hyperparasitized (Fig. 1d).

Figure 1.
Symptoms of Perilla rust and the hyperparasitism phenomenon in field.
(a) Adaxial (upper) leaf surface showing early-stage Coleosporium plectranthi infection. (b) Abaxial (lower) leaf surface with early-stage rust pustules. (c) Mature uredinia of C. plectranthi on the abaxial leaf surface. (d) Hyperparasitism of rust pustules by Ramularia coleosporii.Morphological characteristics of Perilla rust fungus and the hyperparasite fungus
-
The urediniospores of Perilla rust fungus (C. plectranthi) are spherical, ovoid, or pyriform in shape, with uniform size of 14.84~20.43 µm × 14.18~19.64 µm (mean 17.72 ± 1.54 µm × 17.23 ± 1.74 µm). They exhibit an orange-yellow to brown coloration and feature verrucose (wart-like) projections on the surface (Fig. 2a). The conidia of the rust hyperparasite (R. coleosporii) are oblong, elliptical, or short-cylindrical in morphology, with slightly pointed ends, 6~18 µm × 3~5 µm (mean 11.11 ± 2.98 µm × 4.69 ± 0.76 µm) (Fig. 2b).
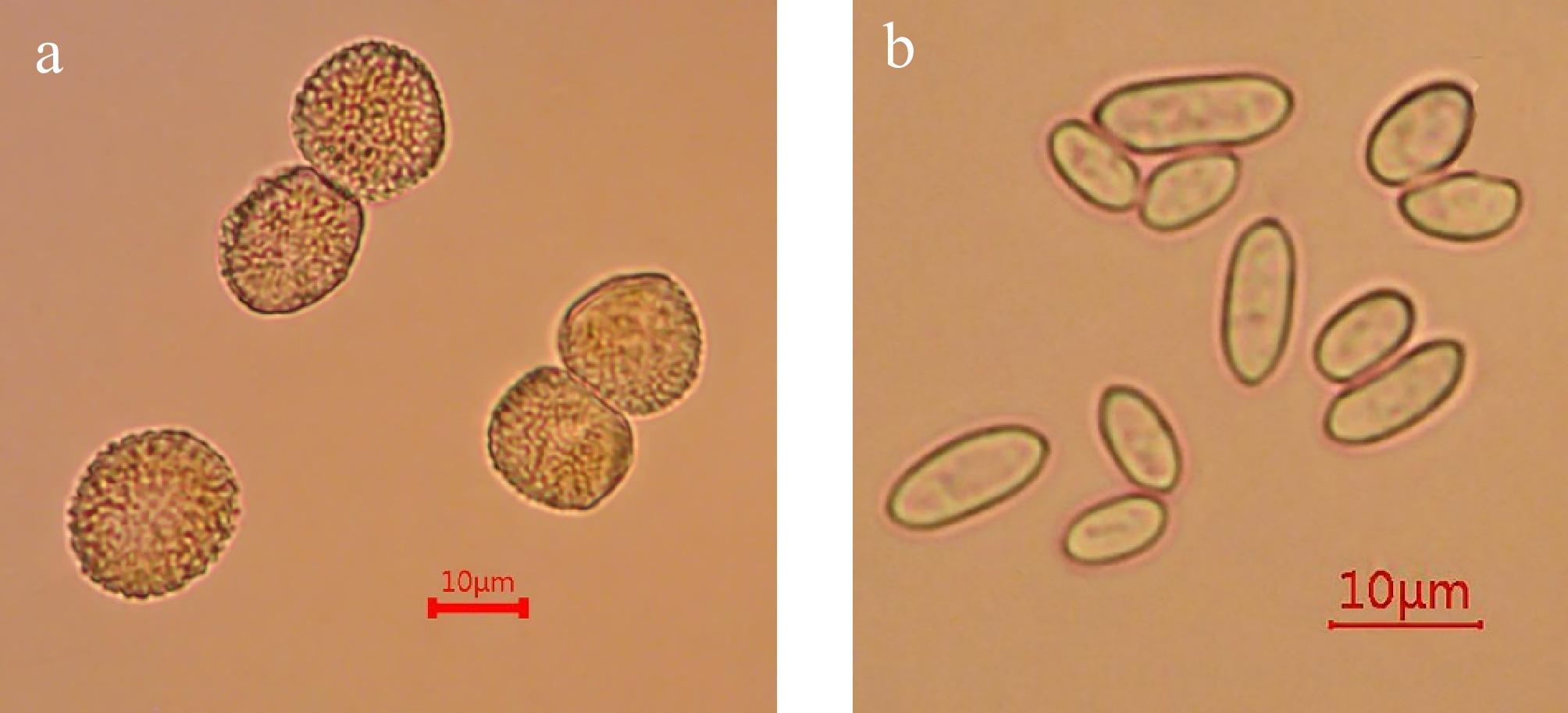
Figure 2.
Morphological characteristics of Perilla rust fungus and its hyperparasitic fungus.
(a) Morphological characteristics of Perilla rust urediniospore. (b) Morphological characteristics of conidia of the rust hyperparasite. Scale bar = 10 μm.Molecular phylogeny of Perilla rust fungus and the hyperparasite fungus
-
The Perilla rust fungus (QXJ1-BJ PV688381; QXJ2-HN PV688382) was amplified using 28S large ribosomal subunit (LSU) rDNA, obtaining two bands of approximately 500-bp. BLAST analysis revealed that the amplified sequence shared 100% identity with C. plectranthi (EF095711) Phylogenetic trees constructed using highly homologous sequences from the GenBank database showed that both Perilla rust isolates clustered in a clade with C. plectranthi (bootstrap support 93%), while exhibiting distant relationships with other Coleosporium species (Fig. 3a). Based on morphological characteristics and molecular sequence alignment, the tested Perilla rust fungus was identified as C. plectranthi.
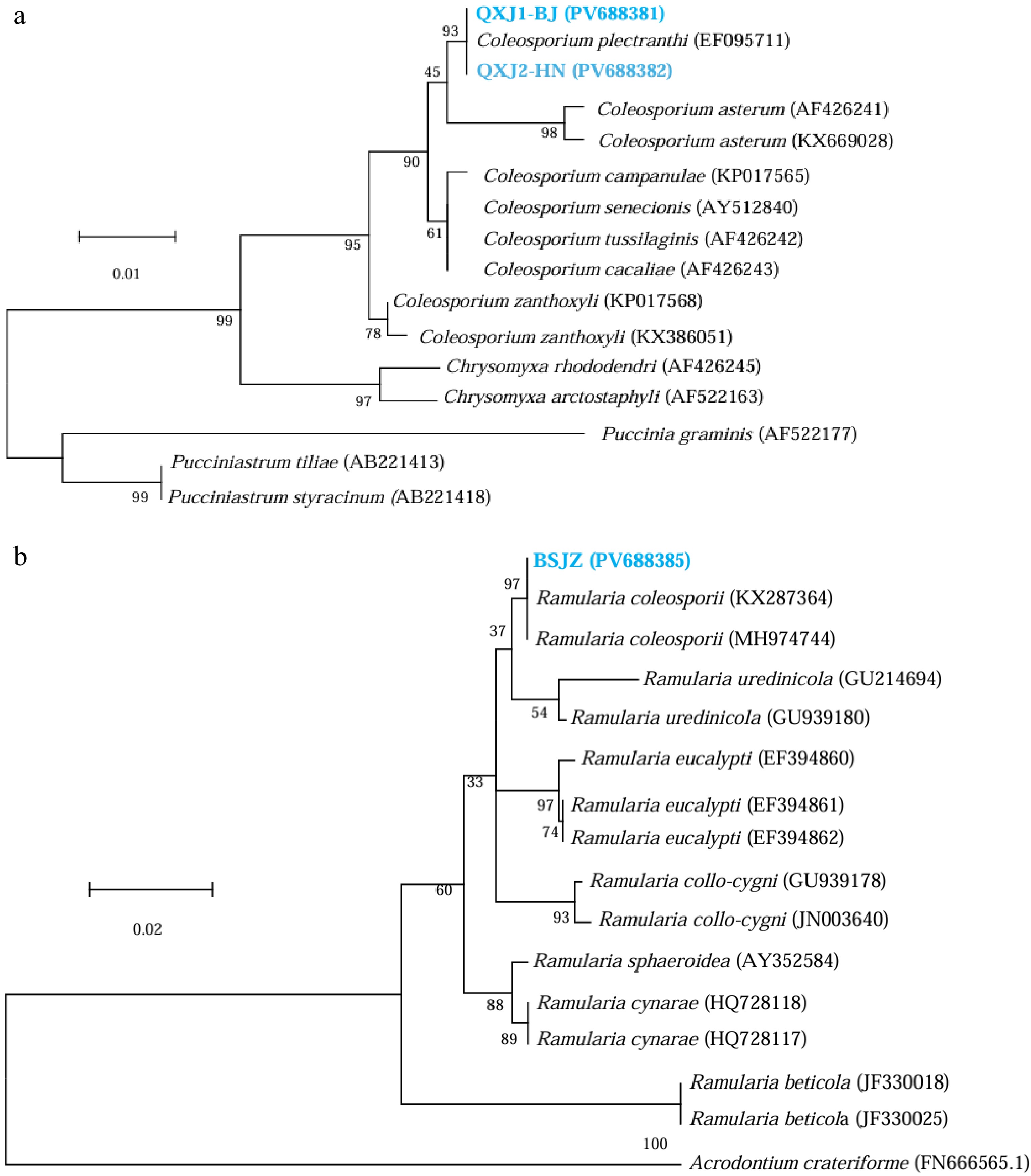
Figure 3.
Phylogenetic trees constructed based on (a) the 28S large ribosomal subunit (LSU) rDNA sequence of Perilla rust fungus, and (b) the ITS sequence of the hyperparasite fungus.
QXJ1-BJ and QXJ2-HN represent Perilla rust fungus from Beijing and Hainan Province (Supplementary Data 1 and 2), BSJZ is the white fungus isolated from the urediniospore pustules of Perilla rust (Supplementary Data 3).The white strain (BSJZ PV688385) yielded a band of approximately 450 bp amplicon using ITS primers. BLAST analysis revealed 100% identity with R. coleosporii (KX287364; MH974744). A phylogenetic tree was constructed using highly homologous sequences from GenBank; the white strain was clustered in a clade with R. coleosporii (bootstrap support 98%), while showing distant genetic relationships with other fungal strains (Fig. 3b). Based on both morphological characteristics and molecular sequence alignment, the hyperparasite of Perilla rust fungus was identified as R. coleosporii.
Pathogenicity and hyperparasitic relationship
-
No white sporulating structures or visible lesions were observed on the abaxial leaf surfaces at 7 dpi of the white fungus inoculated onto healthy leaves of P. frutescens (Fig. 4a). In contrast, when inoculated onto uredinia of rust-infected leaves, a subset of uredinial pustules began whitening at 7 dpi, exhibiting similar symptoms to those observed in the field (Fig. 4c). Over time, the proportion of whitened pustules increased significantly, with the majority of C. plectranthi uredinia becoming colonized by the hyperparasite (Fig. 4d). Perilla rust leaves inoculated with PDA plugs showed no whitening of uredinia, even upon leaf senescence and abscission (Fig. 4b). These results conclusively demonstrate that R. coleosporii is an hyperparasite of C. plectranthi.

Figure 4.
Pathogenicity of R. coleosporii and its hyperparasitic relationship with C. plectranthi.
(a) No lesions were observed on healthy leaves of Perilla frutescens at 7 dpi inoculated with the white fungus under humid conditions. (b) No whitening of pustules observed in the control that were inoculated with PDA plugs near rust uredinia until leaf abscission. (c) Whitening of pustules were observed at 7 dpi that were inoculated with the hyperparasite fungus. (d) Most uredinia inoculated with the hyperparasite fungus turned white later. -
Rust fungi belong to the kingdom of fungi, phylum Basidiomycota, class Pucciniomycetes, and order Pucciniales[22]. As one of the most diverse groups within Basidiomycota, this order comprises approximately 31,515 described species and is widely distributed across natural and agricultural ecosystems[23]. Globally, rust fungi pose severe threats to crops (e.g., wheat, soybean) and tree species used for timber production and bioenergy (e.g., poplar, eucalyptus, pine)[24]. Through long-term coevolution with host plants, rust fungi have developed high host specificity and require living host tissues to complete their life cycle[25].
Pathogens of the genus Coleosporium (Pucciniales: Coleosporiaceae) are known to cause forest diseases such as pine needle rust[26], and Phellodendron leaf rust[27], as well as rust diseases in medicinal plants, including Zanthoxylum[17], Solidago[28], Dendrobium officinale[29], Aster tataricus[30], and Bletilla striata[31]. Although C. perillae has been reported as the causal agent of Perilla rust in China[13], however, no systematic research was published. Some scholars have noted that C. perillae and C. plectranthi exhibit highly similar morphological characteristics[32]. As is well established, morphological characteristics alone cannot definitively determine species information. Thus, molecular investigations were further pursued. However, the lack of 28S large ribosomal subunit (LSU) sequence data for C. perillae has hindered definitive differentiation. In this study, both morphological characteristics and LSU sequences of the collected Perilla rust specimens showed 100% congruence with C. plectranthi. Therefore, it is concluded that the causative agent of Perilla rust in China is C. plectranthi, representing the first systematic report on this pathogen.
Based on sequence alignment and morphological characteristics, this study confirmed that the white fungal strain potentially exhibiting hyperparasitism on Perilla rust fungus was R. coleosporii. Previous studies have reported this species as a causal agent of leaf spot in P. frutescens[18]. Pathogenicity tests revealed that inoculation of the white fungus onto multiple healthy Perilla leaves failed to induce obvious symptoms, with only one leaf developing a light brown necrotic spot at the inoculation site, but didn't show further expansion. This indicates a weak pathogenicity of this strain toward Perilla leaves. However, when inoculated onto uredinial pustules of Perilla rust, all treated rust pustules turned white, which were consistent with those observed in the field. These results conclusively demonstrate that the white fungus functions as a hyperparasite of the Perilla rust fungus. The objective of this experiment was solely to verify whether R. coleosporii acts as a hyperparasite of C. plectranthi. This study has limitations: further investigation is required to determine whether this hyperparasite can extensively suppress the proliferation and progression of Perilla rust, as well as its probability of disease suppression. The authors present only preliminary findings here, serving as a foundational reference for subsequent research, with the aim of contributing to the development of biocontrol strategies against Perilla rust.
Rust disease stands as one of the most significant threats to Perilla production in China. The pathogen causes severe epidemics in major cultivation areas, frequently resulting in premature leaf desiccation and abscission. Even mildly infected leaves become densely covered with uredinial pustules, substantially discounting both yield and leaf quality, especially for fresh consumption. Notably, the harvest window for fresh Perilla leaves coincides with peak rust incidence, rendering chemical fungicides unsuitable for disease management. It is advised to accelerate the breeding of Perilla varieties with rust resistance, while integrating field management practices and rational fertilization to effectively mitigate the occurrence and damage caused by Perilla rust. Furthermore, this study not only identified the pathogenic rust fungus in China, but also discovered a hyperparasitic strain capable of colonizing the rust pathogen without causing apparent harm to Perilla leaves, suggesting its potential for biological control of Perilla rust. Future efforts should explore biological control strategies and host resistance induction to enable early prevention of Perilla rust, thereby achieving scientifically sound and efficient disease management to ensure steady improvements in Perilla yield and quality.
-
The pathogen causing Perilla rust in China is C. plectranthi, and the white fungus hyperparasite is R. coleosporii.
This work was supported by grants from the National Key R&D Program of China (2022YFC3501504), and the CAMS Innovation Fund for Medical Sciences (2021-I2M-1-032).
-
The authors confirm contributions to the paper as follows: study conception and design: Li Y; data collection: Wang LY, Zhong S, Wang B; analysis and interpretation of results: Wang LY, Zhong S; draft manuscript preparation: Li Y. Proof reading and editing: Zhong S, Wang LY, Gao WW; funding acquisition, supervision, resource: Wei JH, Li Y. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
-
The authors declare that they have no conflict of interest.
-
#Authors contributed equally: Ling-Yu Wang, Shan Zhong
- Supplementary Data S1 QXJ1-BJ (PV688381).
- Supplementary Data S2 QXJ2-HN (PV688382).
- Supplementary Data S3 BSJZ (PV688385).
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Yunnan Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang LY, Zhong S, Wang B, Gao WW, Wei JH, et al. 2025. Investigation of the occurrence of Perilla rust and its hyperparasitism in field and molecular and micro-morphological identification of their causal agents in China. Agrobiodiversity 2(4): 73−77 doi: 10.48130/abd-0025-0010
Investigation of the occurrence of Perilla rust and its hyperparasitism in field and molecular and micro-morphological identification of their causal agents in China
- Received: 30 May 2025
- Revised: 14 October 2025
- Accepted: 22 October 2025
- Published online: 05 November 2025
Abstract: Perilla frutescens (L.) Britt. is widely cultivated in China for its medicinal and culinary uses. Rust disease is prevalant, and hyperparasitism was usually observed in Perilla producing regions annually. The urediniospores of Perilla rust fungus are spherical, ovoid, or pyriform, and the conidia of its hyperparasite are oblong, elliptical, or short-cylindrical. A pure culture of the hyperparasite was isolated using the single spore isolation method. The 28S large ribosomal subunit rDNA gene (nrLSU) of rust fungus, and the internal transcribed spacer region (ITS-rDNA) of the hyperparasite were amplified. These two fungi shared 100% identity with Coleosporium plectranthi and Ramularia coleosporii. Phylogenetic trees showed that whole Perilla rust isolates clustered in a clade with C. plectranthi, while the hyperparasite fungus clustered in a clade with R. coleosporii, with a bootstrap support value of 93% and 98%. Combining micro-morphology and molecular sequence alignment, the rust fungus and its hyperparasite were identified as C. plectranthi and R. coleosporii. Urediniospore pustules of C. plectranthi gradually turned white after inoculation of R. coleosporii, while pustules inoculated with PDA plugs showed no change, which confirmed their hyperparasitic relationship. This study determined C. plectranthi as the causal agent of Perilla rust in China, and R. coleosporii as its hyperparasite.
-
Key words:
- Coleosporium plectranthi /
- Perilla frutescens /
- Rust disease /
- Hyperparasitism /
- Biocontrol













