-
Hydrogen peroxide (H2O2) is a green oxidizer which is widely used in wastewater treatment, disinfection, bleaching, and chemical synthesis. The anthraquinone method dominates the current industrial production of H2O2, which is energy-intensive and poses significant environmental risks. In contrast, photocatalytic production of H2O2 emerges as an environmentally friendly approach, since it relies solely on readily available resources, such as the abundant water, oxygen, and sunlight on Earth[1]. In particular, the light-driven generation of reactive oxygen species (ROS), such as superoxide (•O2−), singlet oxygen (1O2), or hydroxyl radicals (•OH) can drive in situ oxidation reactions for the synthesis of value-added chemicals. Coupling photocatalytic H2O2 generation and aerobic oxidation is highly desired but has rarely been reported. Covalent organic frameworks (COFs) are emerging catalysts for photocatalytic H2O2 production because of their customizable structure and functional sites[2]. However, the most extensively studied imine-linked COFs have several challenges as photocatalysts, including low chemical stability, unsatisfactory long-term photocatalytic stability, discontinuous π-conjugation, insufficient charge dissociation, and sluggish charge transfer[3]. Rational design of the conjugation structure and catalyst sites in COFs offers a promising strategy for promoting photocatalytic H2O2 generation and chemical synthesis.
The recently reported work by Yang et al.[4] demonstrated the synthesis of thiazole-based homologous heteropolyaromatic COFs (TTT-COFs) through the sulfur-vapor-mediated post-cyclization for enhanced photocatalytic H2O2 production and aerobic oxidation of C(sp3)-H bonds (Fig. 1a). The post-cyclization reaction and sulfur dosage were optimized to guarantee complete cyclization and maintain the crystalline and chemical structure. The introduction of thiazole linkage creates several advantages as follows: (1) Exceptional robustness under harsh chemical conditions; (2) continuous π-conjugation of TTT-COFs guarantees efficient single-electron transfer (SET) and energy transfer (EnT) for photocatalysis, significantly enhancing the intramolecular polarity for boosting charge separation and transfer (Fig. 1b); (3) promotion of the adsorption activation of O2 intermediates in oxygen reduction reactions (Fig. 1c). Impressively, TTT-COFs deliver a H2O2 evolution yield of 29.9 mmol g−1 h−1 in an aqueous solution with 10% benzyl alcohol (v/v) as a sacrificial agent under visible light and O2 purging. The performance is 20× higher than its imine-linked counterpart and rivals that of the best organic (e.g., the protonated covalent triazine polymer [TTH–CTP]; 23.7 mmol g−1 h−1) or inorganic photocatalysts (e.g., Na doped g- C3N4 enriched with cyano group, the mass ratio of NaCl to cyano precursor is 5 [5Cv@g-C3N4]; 7.010 mmol g−1 h−1) reported to date. In pure water, the H2O2 yield was 4.75 mmol g−1 h−1. This result indicates that benzyl alcohol (BA) could strongly suppress the decomposition of H2O2. Notably, the unique structure endows TTT-COFs with stable photocatalytic activity for over 200 h of continuous operation with negligible decay (Fig. 1d). More importantly, under mild conditions driven by visible light and with O2 as the oxidant, TTT-COFs can efficiently and selectively achieve aerobic oxidation of ethylbenzene and its derivatives, with a maximum conversion rate of up to 99%, as well as wide substrate applicability and excellent cycling stability (Fig. 1e). A study of the mechanisms reveals that 1O2 and •O2− generated in situ synergistically contribute to the aerobic oxidation of C(sp3)-H (Fig. 1f). The complete thiazolization is the key switch to turn on 1O2 generation, and the double ROS effect makes the TTT-COF a rare, metal-free, visible light-driven, and recyclable photocatalyst. In summary, this work highlighted the facile post-synthetic functionalization strategy to synthesize robust COF-based photocatalysts with customizable properties for highly efficient H2O2 photosynthesis and in situ aerobic oxidation reactions.
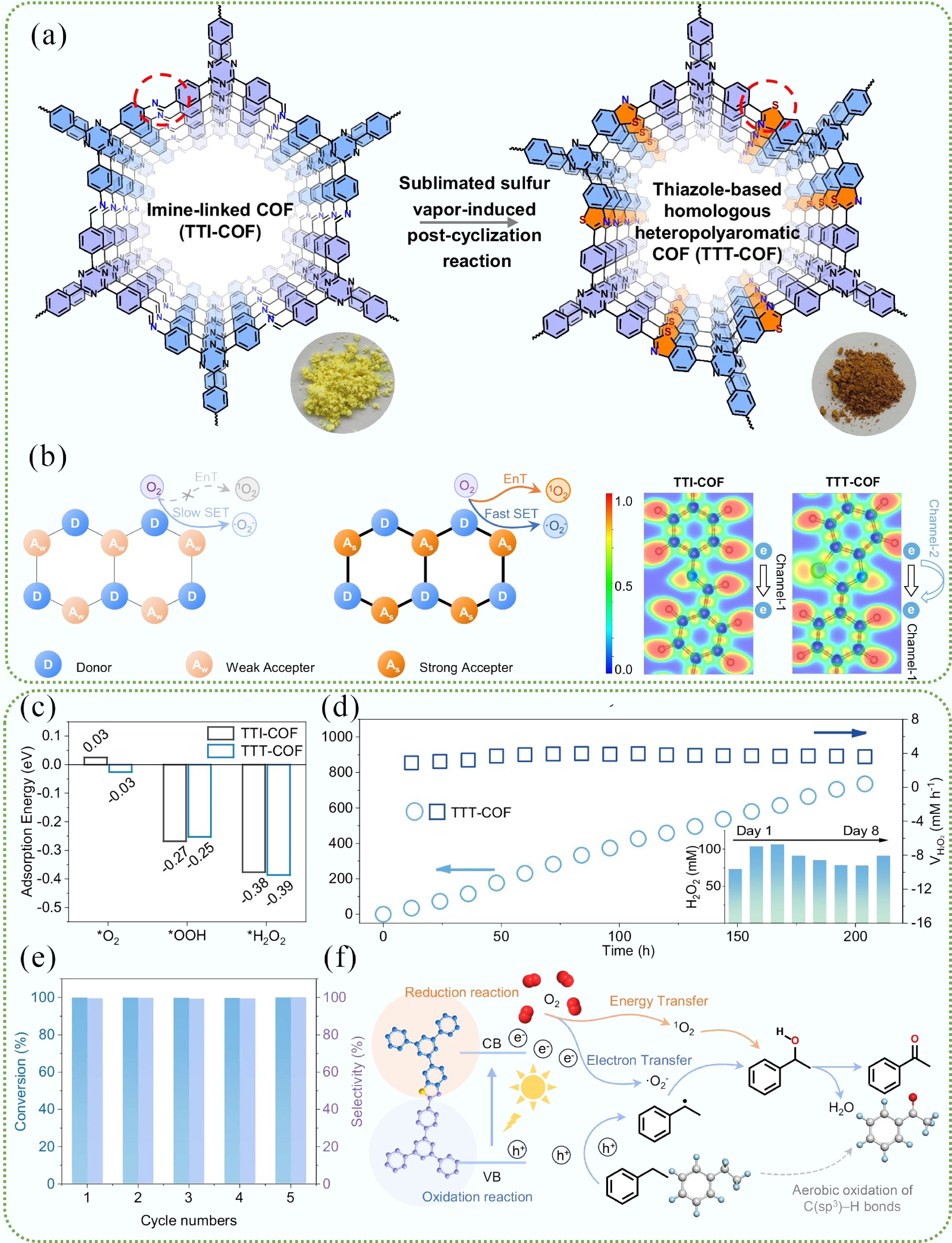
Figure 1.
(a) Synthesis of a triphenyl triazine thiazole COF (TTT-COF) from triphenyl triazine imine COF (TTI-COF) via the post-cyclization reaction. (b) Left: Schematic illustration of the structural advantages of the homologous heteropolyaromatic COF (TTT-COF). Right: Calculated electron localization functions (ELF) diagrams of the TTI-COF and TTT-COF. (c) The adsorption energy of the TTI-COF and TTT-COF with oxygen-containing intermediates. (d) Long-term photocatalytic H2O2 production of the TTT-COF (the inset shows the yield of H2O2 production per day over an eight-day period). (e) Cycle experiment of aerobic oxidation of ethylbenzene along with the TTT-COF as the photocatalyst. (f) Proposed mechanism for the aerobic oxidation of C(sp3)-H bonds[4].
This pioneering exploration of chemically stable COFs in aerobic oxidation reactions through ROS generated in situ inspires the design of next-generation photocatalysts for coupling photocatalytic H2O2 production with other reactions such as biomass conversion, organic synthesis, and pollutant degradation. The coupled reaction systems could maximize solar energy utilization and also deliver multiple value-added products. Notably, the development and practical application of COF-based photocatalysts for highly efficient H2O2 synthesis and coupled photocatalytic process are still in their infancy and pose several challenges. First, the multi-step synthesis and modification processes under elevated temperatures with the use of expensive monomers and additional chemicals incur high costs and time consumption in scaled-up production. Second, the limited charge separation and transfer properties, alongside the light-harvesting capability of COFs make it difficult to maintain high efficiency under open-air conditions and real sunlight in the absence of sacrificial agents. Third, the powdered COF photocatalysts are difficult to deploy in practical applications, and their structure and functional performance frequently deteriorate during scaled-up production. Future advances will require concerted efforts in material design, mechanistic understanding, and engineering for practical deployment. First, it is essential to establish the correlations between the structural parameters and the resulting photocatalytic properties by taking advantage of theoretical methods, including density functional theory (DFT), molecular dynamics, and machine learning. Utilizing theoretically predicted innovative design strategies to guide the fabrication of high-performance photocatalysts through advanced experimental methods can reduce the costs and time consumption of trial and error. Second, we can deepen the mechanistic understanding of ROS generation and conversion pathways in coupled reaction systems through advanced in situ characterization and computational modeling. Third, we need to develop robust photocatalysts with a free-standing structure, achieving mass production through a solvent-free method, and solving the practical engineering issues, including photocatalyst deployment and continuous flow reactors. Adopting this strategic roadmap will accelerate the transition of COF-based photocatalysis from a promising laboratory concept to scalable industrial method that delivers greener chemical manufacturing and a more sustainable energy future.
HTML
We acknowledge the financial support from the National Natural Science Foundation of China (22176055).
-
The authors confirm their contributions to the paper as follows: Bing Han: Conceptualization, writing - original draft, writing - review & editing, funding acquisition; Yin Zhang: writing - review & editing. Both authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interests.
-
Full list of author information is available at the end of the article.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Han B, Zhang Y. 2025. Rational design of robust catalysts for enhanced photocatalytic hydrogen peroxide production and value-added chemical synthesis. Sustainable Carbon Materials 1: e009 doi: 10.48130/scm-0025-0008 |












