Background: Sorafenib has consistently served as the control arm in multiple randomized clinical trials (RCTs) evaluating novel therapies for advanced hepatocellular carcinoma (HCC) for more than a decade. Analyzing trends in clinical outcomes of patients treated with sorafenib for the same indication over time offers the opportunity for unique insight into the evolution of clinical trial conduct and potential non-drug factors impacting outcomes.
Methods: We identified RCTs in patients with treatment-naïve advanced HCC where sorafenib was compared to another systemic therapy or placebo. We extracted trial-level demographic, clinicopathologic, and outcome data (overall survival [OS], progression-free survival [PFS], objective response rate [ORR], and duration of therapy). Sample-weighted linear regression was used to identify temporal trends with significance set at p ≤ 0.05.
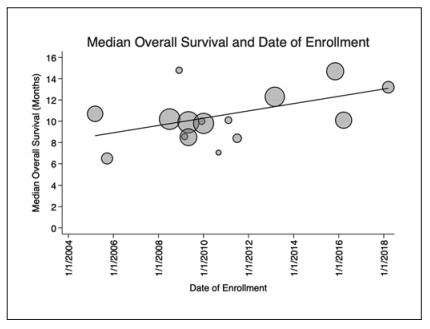
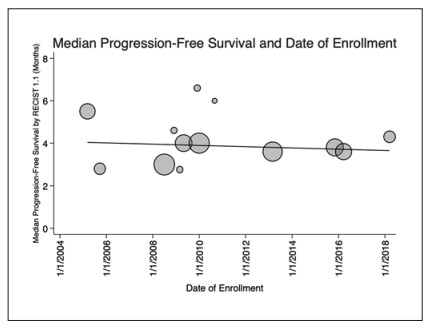
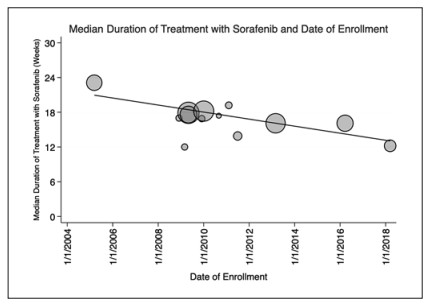
Results: Sixteen RCTs (9 phase Ⅲ and 7 phase Ⅱ) enrolling 4,086 patients treated with sorafenib were included in the analysis. Included trials enrolled patients from 2005 to 2019. OS has significantly improved by 4.5 months from 2005 to 2019 (p = 0.048) over time. Thirteen studies provided data on PFS using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, with no significant change over time (p = 0.69). ORR assessed by RECIST 1.1 has significantly improved by 6.0% over time (p = 0.003). Median duration of therapy with sorafenib has decreased by 53% since the enrollment of the first clinical trial in 2005, from 23.1 weeks to 12.2 weeks (p = 0.0037). There was no significant change in patient demographics were identified over time to explain the OS findings.
Conclusion: The median OS of patients with advanced HCC treated with sorafenib has improved significantly over 15 years. At the same time, the median duration of therapy with sorafenib has decreased. The reason for these findings was not explained by changing demographics of patients enrolled in these trials and has implications for ongoing clinical trials.