-
Ornamental geophytes contain more than 800 different botanical genera which have different development biology and physiology. Morphologically, ornamental geophytes are characterized by modified organs (stem, root, leaf, or hypocotyl) grown underground and used as storage organs for plant growth and propagation, including tulip, lily, gladiolus, narcissus, hyacinth, freesia, dahlia, hippeastrum, and others. High land plants are evolutionally originated from marine organisms. During the Late Tertiary, the climatic zones were formed and the flora was dominated by woody angiosperms. Land plants have to evolve new survival strategies to cope with climate change. Some species obtained the ability to survive adverse periods by developing underground modified organs. Based on morphology, geophytes are classed into five groups: bulbs (the majority), tuber, corm, rhizome, and tuberous roots (Fig. 1). Geophytes are major players in the international flower industry and are widely used as cut flowers, potted plants, landscaping, and gardening plants. Most of the geophytes have a growth cassation period (called dormancy) except species that are originally from tropical areas, like Crinum asiaticum, Hippeastrum rutilum, and Curcuma alismatifolia Gagnep.

Figure 1.
Photos to document the classification and morphological diversity of geophytes. (a) Dormant lily bulbs, (b) gladiolus corms, (c) Zantedeschia aethiopica tuber, (d) Canna indica rhizome, (e) Dahlia tuberous root. ab: apical bud; ax: axillary bud; bd: bud; bp: basal plate; br: branch root; cr: root crown; nd: node; rt: root; rz: rhizome; sc: scale; st: stem root; tr: tuberous root. The scale bar represents 1 cm.
Dormancy is defined as the inability to initiate growth from meristems (and other organs and cells with the capacity to resume growth) under favorable conditions[1]. In bulbs, dormancy could be further divided into three types: i) endodormancy, which is regulated by internal factors. Geophytes in endodormancy are not able to grow even when placed under favorable conditions, e.g., gladiolus and lily; ii) ecodormancy, which is reduced growth response to external signals, like high/low temperature, short photoperiods, drought, and low oxygen. Once the external signals are removed, geophytes resume growth. Non-deciduous bulbs (hippeastrum) have ecodormant phenomena when planted in the temperate zone; iii) paradormancy, which is known as apical dominance. Axil bud growth is inhibited by internal signals[2,3].
Dormancy is an essential trait in the horticultural industry. Pre-sprouting and deep dormancy are problems for crops and geophytes[4,5]. The occurrence of geophyte dormancy restricts the growth period and increases storage costs. On the other hand, the dormancy period allows the commercial handling, storage, and transportation of dormant organs world wide. Therefore, precise regulation of geophyte dormancy and GDR is required for effectively managing their production, shipping, and utilization.
Recently, the topic of perennial woody bud dormancy has been reviewed[3,6−9]. However, few reviews regarding GDR are available. Although both geophytes dormancy and woody bud dormancy belong to bud dormancy or vegetative organ dormancy, several morphological and physiological differences may cause some special responses in geophytes: i) unlike dormant branch buds (e.g., poplar, pear, or apple tree), buds of geophytes are grown on modified storage organs (except tuberous roots where buds grow on the attached root crown) which contain much more starch, glycerol, or sugars than regular branch bud; ii) embedded in soil, dormant geophytes have a few different environmental conditions from regular branch bud, e.g., geophytes may have different strategies to sense the change of light.
In this review, we summarize the current knowledge of GDR and review progress made in the area of environmental and hormone regulation, epigenetics, and miRNA. We also discuss strategies for GDR in the bulb flower industry and prospects for future studies.
-
For bulb plants, the temperature is the most important factor that affects geophytes dormancy (GD). Many geophytes in equatorial and subtropical zones, where there are relatively uniform environmental conditions, rarely have marked rest periods and continuously develop foliage leaves, such as Hippeastrum, Crinum asiaticum, and Haemanthus multiflorus Martyn.. However, when planted in marked climatic changes conditions, non-deciduous bulbs exhibit ecodormant phenomena, such as Hippeastrum and Clivia. Geophytes located in the Mediterranean go into dormancy in hot summers suggesting temperature is an essential factor controlling GD. When the temperature drops in the temperate zone, most bulb plants go into dormancy from autumn and are not suitable for plant growth, such as Begonia tuberhybrida, Tigridia pavonia, and Gladiolus hybridus. In the flower production chain, bulbs were stored at a relatively low temperature to extend ecodormant duration when undergoing long-distance shipping and forcing flowers throughout the year, such as tulip (storing at −2 to 9 °C), lily (at −2 to −1 °C), and gladiolus (at 4 °C).
Temperature signaling serves as a critical environmental cue for GDR (Fig. 2). Besides, temperature signal interplays photoperiod signals in perennial dormant vegetative organs[10]. In recent years, a cold sensor (COLD1; CHILLING-TOLERANCE DIVERGENCE1) is identified in rice, which interacts with G protein to activate the Ca2+ channel for temperature sensing[11]. In temperature signaling, ICE1 (INDUCER OF CBF EXPRESSION), an MYC-like bHLH transcriptional activator, is induced by low temperature and promotes both seed dormancy in Arabidopsis and bud dormancy in poplar, pear, and apple[12−14]. Besides, by using transcriptome analysis, ICE1 is suggested to be involved in bud dormancy of geophytes, like leafy spurge, gladiolus, and lily, suggesting its conserved role in regulating GD[15−17]. ICE1 could bind to CBFs (C-REPEAT/DRE BINDING FACTORs) and active DAM (Dormancy-Associated MADS-Box) expression, promoting endodormancy[18,19]. DAM was firstly characterized in a peach mutant (evergrowing) which exhibits constant growth without dormancy[20]. DAM is a MADS-box protein and homologous to SVP (SHORT VEGETATIVE PHASE) in Arabidopsis, which binds the CArG motif of the FT (FLOWER LOCUS T) promoter and directly inhibits FT expression[21]. FT has been well characterized in perennial bud dormancy that it plays a positive role in bud dormancy release, like Gentian and poplar[22,23]. DAM is involved in regulating bud dormancy of trees by repressing GA biosynthesis and cell division, and promoting callose accumulation near plasmodesmata (PD)[24].
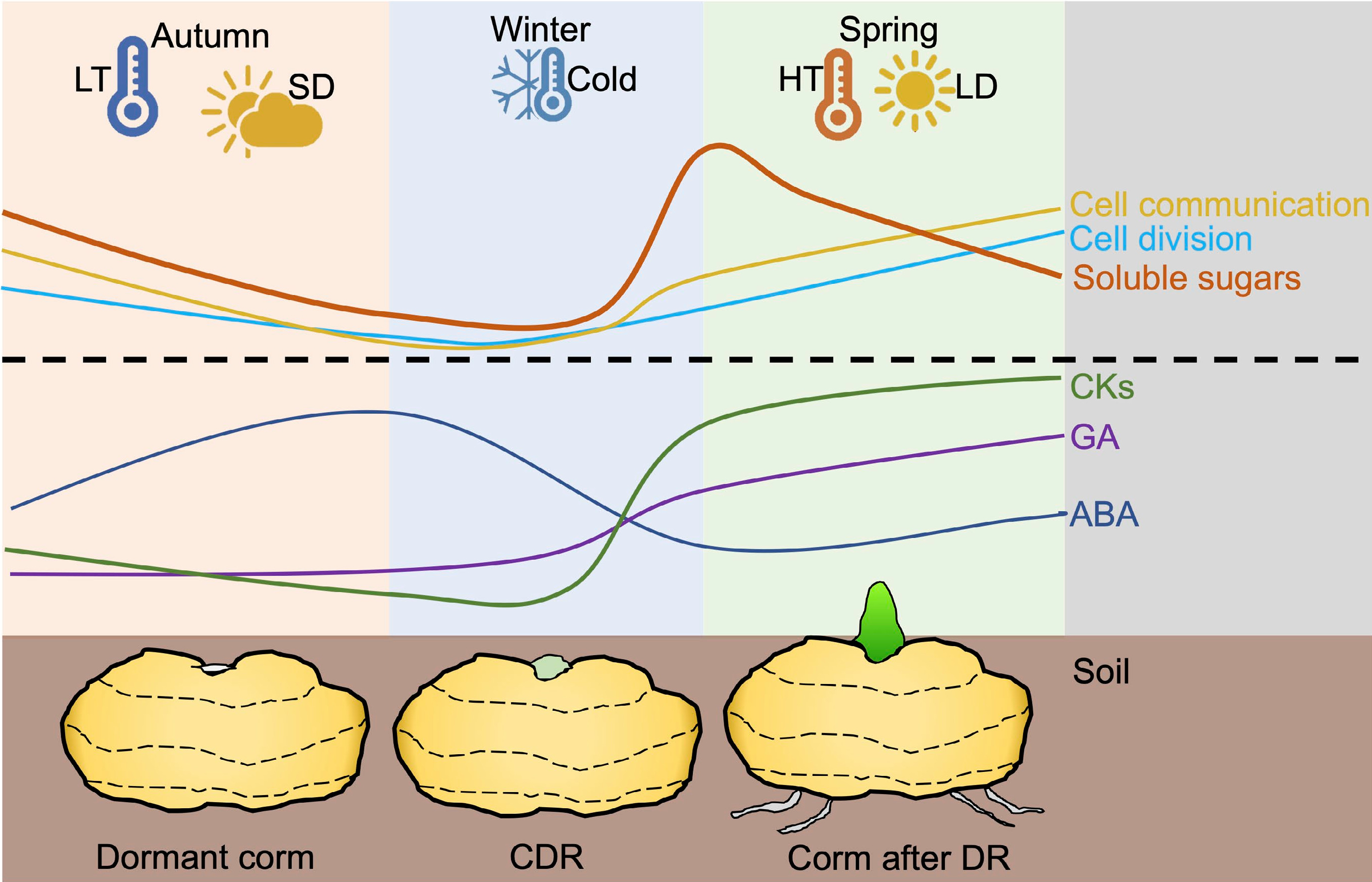
Figure 2.
Biochemical and physiological processes occur during the whole process of dormancy in geophytes. In autumn, low temperature and short days induce endogenous ABA and inhibit CKs and GA. Besides, soluble sugars are decreased in shoots along with blocked PD and slow cell division. During the winter, long-term cold treatment contributes to decreasing ABA content in dormant organs and promoting GA, CKs, and soluble sugars. Meanwhile, callose around the PD is degraded which helps to active cell communication and cell division in buds. When the temperature and light are suitable for corm sprouting, ABA is continuously decreased and soluble sugars are used for plant growth. CDR: corm dormancy release; DR: Dormancy release; LD: Long day; LT: low temperature; SD: short day.
Besides ICE1, ambient temperature is also sensed by phytochrome B (phyB) in Arabidopsis[25]. The active phyB is involved in plant growth and development including dormancy[24,26]. The photoconversion between Pr and Pfr enables the connection of the environmental cues with morphological reactions or plant behaviors[24]. It is possible that phyB can also regulate GDR when soil temperature changes, even if there is no light.
In the actual planting of geophytes, to accelerate GDR, methods like warm baths and cold storage are widely used[27]. However, the mechanism of warm baths for geophytes is less known, compared to cold storage.
Light
-
Light is another important environmental factor affecting GD and GDR mainly by its light quality, quantity, and duration. In autumn, before the temperature decreases dramatically, light duration is much shorter than in the summer (Fig. 2). Plants can sense light changes with sensors, e.g., phyA, phyB, CRY1, and CRY2[28,29]. Light participates in dormancy induction of geophytes, however, it might have a weaker effect on GDR compared to temperature. Because dormant geophytes were imbibed in the soil naturally.
It has been shown that different light wavelength has different effects on plant development including vegetative growth, flowering, and dormancy[30]. Light with a specific wavelength is recognized by a certain light sensor[30]. ULTRAVIOLET B RESISTANCE 8 (UVR8) senses UVB light (280−315nm); Cryptochrome (cry), phototropin (phot), ZEITLUPE (ZTL), and phytochrome (phy) can sense light with 315−500 nm wavelength; in addition, phytochrome can sense red and far-red light[31]. phyA is active in darkness (the very low fluence response; VLFR) and the R/FR high irradiance response (R/FR‐HIR) while it maintains low levels in the white light. phyB is abundant and stable under white and red light. There are two forms of phyB in plants: one is Pr (inactive form) and the other is Pfr (active form). Pr is converted into Pfr by absorbing R light while Pfr is converted into Pr by absorbing FR light[32]. In the light response, phyA and phyB inhibit PIFs (PHYTOCHROME‐INTERACTING FACTORS) and further regulate cell division and cell elongation[33,34]. Although there is limited research about light-regulated GDR, several studies have shed light on light-regulated bud and seed dormancy[33,35]. In Populus, the phyB-PIF8 module responds to the light changes and controls seasonal growth cessation and bud break by cell division[33]. In Arabidopsis seeds, the phyB-PIF1 module mediates seed dormancy and germination by regulating the antagonism between GA and ABA metabolism[35]. In barely, white light and blue light had dramatically stimulation of HvNCED1 expression dry dormant seed to increase the ABA content to maintain the dormancy[36]. Moreover, compared with the monochromatic photoreceptors, multiple photoreceptors, i.e., the combination of blue and green light photoreceptors, play a more efficient role in the maintenance of dormancy in imbibed seeds of Lolium rigidum[37].
In addition, light quantity and duration control GDR in species such as lily, potato, Anemone coronaria, and Lotus[38−41]. In Easter lily, dormancy and vernalization could be achieved by combining long-day photoperiods and cold treatment, which can be technically replaced by each other equally on a week-for-week basis[42]. For most winter dormant geophytes, short day (SD) conditions result in growth cessation and endodormancy, e.g., Gladiolus, Asiatic lily, Hosta plantaginea[27]. SD is sensed by phytochromes in plants and mediates endodormancy by regulating CONSTANS (CO)/FT expression[24]. In addition, SD also induced endogenous ABA levels by stimulating DAM expression[24].
-
Hormones are the most effective regulators in regulating plant growth and development as well as dormancy[24]. It is well known that ABA is the master hormone in the whole process of GD (Fig. 3), including dormant induction, maintenance, and release[24]. We have discussed the mechanism in our previous review, containing the interplay between ABA and environmental factors, hormones, sugars, or epigenetics[24]. In Brief, ABA is involved in dormancy by repressing cell division, decreasing energy transition, blocking cell communication via PD, and slowing down the transcription and translation of genes.
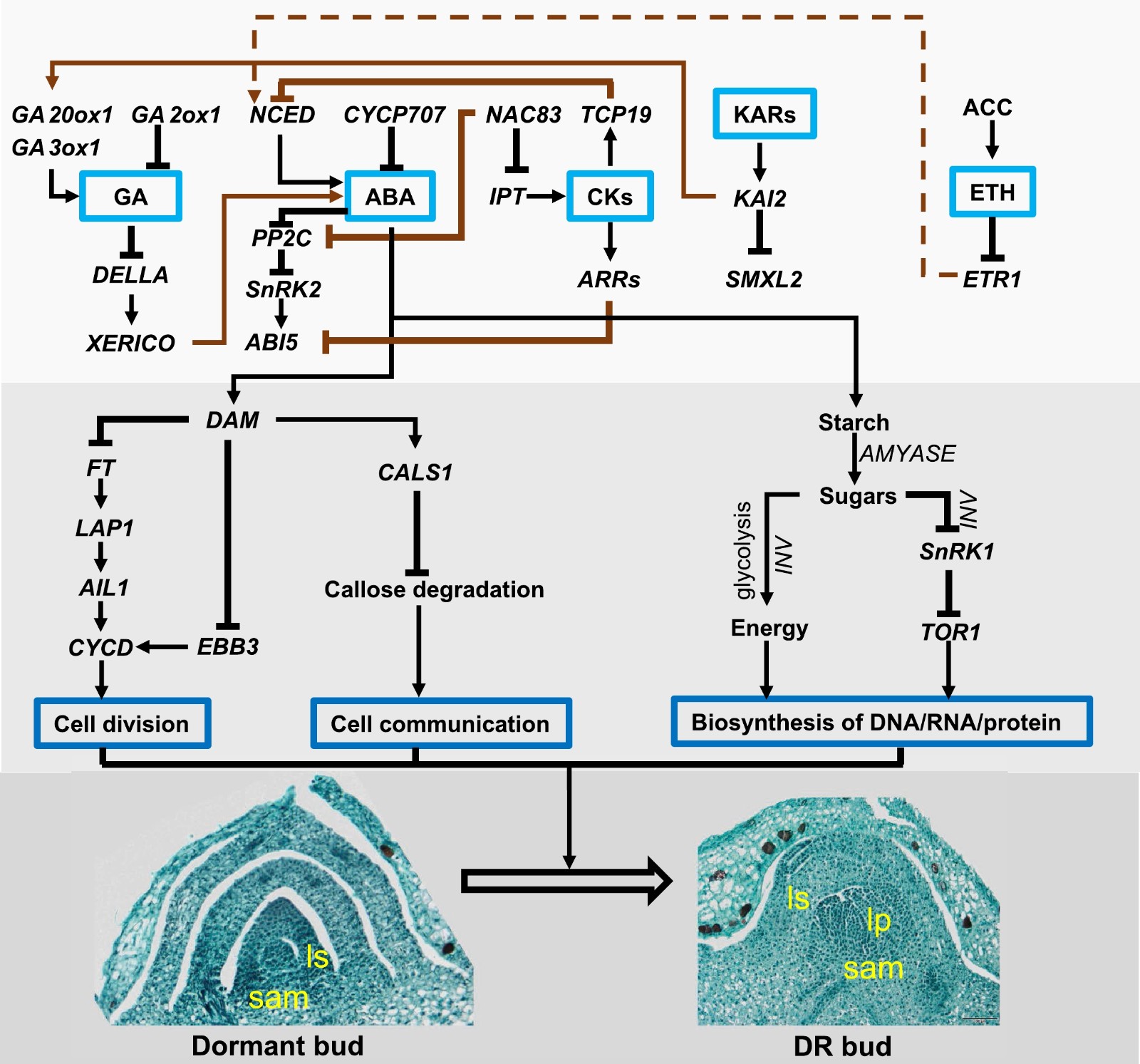
Figure 3.
The interplay among hormones and carbohydrates in regulating GDR. During GDR, ABA is the master hormone that could delay dormancy release by repressing cell division, blocking cell communication via callose, and inhibiting the biosynthesis of DNA, RNA, and protein by releasing SnRK1. Other hormones like GA, CKs, KARs, and ETH could interplay with ABA via transcription factors. A solid line represents the direct effect, and a dashed line represents the indirect effect. The interplay is summarized from different species. ABA: abscisic acid; ABI5: ABA INSENSITIVE 5; ACC: 1-Aminocyclopropane-1-carboxylic acid; AIL1: AINTEGUMENTA-like 1; ARR: RESPONSE REGULATOR 1; CALS1: CALLOSE SYNTHASE 1; CKs: cytokinins; CYP707A: CYTOCHROME P450, FAMILY 707, SUBFAMILY A; CYCD: D type CYCLINS; DAM: Dormancy-Associated MADS-Box; DELLA: aspartic acid–glutamic acid–leucine–leucine–alanine; EBB3: EARLY BUD-BREAK 3; ETH: ethylene; ETR1: Ethylene Receptor 1; FT: FLOWER LOCUS T; GA: Gibberellic acid; GA2ox1: Gibberellin 2-Oxidase 1; GA20ox1: Gibberellin 20-Oxidase 1; GA3ox1: Gibberellin 3-Oxidase 1; IPT: ISOPENTENYL TRANSFERASE; KARs: Karrikins; KAI2: KARRIKIN-INSENSITIVE 2; LAP1: Like- APETALA 1; NAC: NAM, ATAF, CUC; NCED: 9-CIS-EPOXYCAROTENOID DIOXYGENASE; PP2C: Protein phosphatase 2C; SnRK1/2: SNF1-related protein kinase 1; TCP: Teosinte Branched Cyldoeia/PCF; TOR1: Target of Rapamycin; SMXL2: SMAX1-LIKE.
Gibberellic acid
-
For several geophytes, GA is also considered an essential hormone in promoting GDR (Fig. 3), e.g., tulip, Oriental lily, and Fritillaria meleagris[43−45]. However, it is not always the case in Gladiolus, Polianthes tuberosa, and others[5,46].
During natural GDR, endogenous GA is induced by long-term cold exposure and geophytes are ready to sprout when spring arrives[27]. During bud dormancy release, chilling repressed SVL expression, and thus up-regulates the transcript of SVL'S target genes, like GA20ox[47]. Increased GA could be an antagonist to ABA and bud dormancy release by promoting cell division, soluble sugar content, energy metabolism, and reopening plasmodesmata communication[24,48,49]. Negative regulators in GA signaling, DELLA proteins, are degraded along with increased GA content during bud dormancy release in tree peony and Japanese apricot[50,51].
Cytokinins
-
In geophytes, cytokinins (CKs) turn out to be an efficient hormone to break endodormancy and paradormancy (axillary bud dormancy), e.g., Gladiolus, Zantedeschia, Narcissus, and Fritillaria meleagris[17,45,52,53]. Endogenous CKs are stimulated by chilling and play a positive role in GDR (Fig. 3).
During GDR, CKs biosynthesis genes including isopentenyl transferase (IPT) and CYP735As (CYTOCHROME P450, FAMILY 735, SUBFAMILY As) are active while CKs inactive pathway is inhibited, such as CKP1 (CYTOKININ RIBOSIDE PHOSPHORYLASE1)[17,54]. Silencing either of these genes results in a short dormant period in Gladiolus corm and potato tuber[17,55]. The functions of CKs are as follows: 1) promoting cell proliferation and division via cell cyclin genes. In Gladiolus, accumulated CKs promote the transcripts of CYCLIN genes[2]. In potato tubers, dormant cells are arrested at the G1 cell stage, and CYCLIN D members are involved in active this process[55]; 2) enhancing secondary PD formation and PD transport in shoot meristem[56,57]. Silencing CK receptor genes (AHK3 and AHK4) reduces PD transport[57]; 3) antagonizing ABA during GDR. CKs inhibit ABA biosynthesis and signaling transductions via transcription factors during GDR, including NAC83, SVL, and TCP19 (Teosinte Branched Cyldoeia/PCF) in geophytes[2,17,24].
Ethylene
-
Ethylene is a small molecular gaseous hormone and several research articles have shown that it is involved in GDR in onion and Liatris, among others (Fig. 3)[58,59]. Similar to geophytes, in birch, ethylene is induced under SD conditions and inhibits cell division in shoot meristem which further contributes to bud dormancy[60]. Mutating ethylene response (ETHYLENE RESPONSE1) results in decreasing response to SD and delayed endodormancy in birch as ABA accumulation and signal transduction are abolished in etr1 mutant[60]. In chrysanthemum, ethylene is induced by cold temperatures and triggers growth cessation and dormancy. The etr1 mutant in Chrysanthemum is insensitive to ethylene and has a delayed dormancy phenotype[61]. In onion, exogenous ethylene upregulates ABA biosynthesis and delays bulb dormancy[58].
However, the role of ethylene on GDR is various when the situation or species changes. In Gladiolus, the ethylene-releasing compound (chloroethane-phosphonic acid; CEPA) promotes the sprouting of dormant cormels but inhibits the sprouting of non-dormant cormels[62]. In freesia, ethylene exposure time and repeat times affect the accelerated speed of corm dormancy release[63]. The underlying mechanism still needs to be investigated.
Karrikins
-
Smoke has been used to break dormancy in geophytes since the 1990s, such as freesia and Gladiolus[27,64]. Moreover, smoke is a broadly effective stimulant that enhances the germination of approximately 1200 species in more than 80 genera[65]. Karrikins (KARs) are a group of chemicals that are defined as a kind of plant hormone found in the smoke of burning plant materials[65]. Karrikinolide (butene lactone, 3-methyl-2H-furan[2,3-c]pyrone, KAR1) was first identified in 2003 and characterized as the most effective and abundant germination stimulant among all KARs[66,67]. Although there is no direct evidence that shows KARs promote GDR whereas it seems that KARs possibly have a conserved role in the seed germination of crops and weeds[68].
KARs trigger the association of KAI2 (KAR receptor) with SMXL2 (SMAX1-LIKE) and MAX2 (MORE AXILLARY GROWTH2), leading to ubiquitination and degradation of SMXL2, and further promoting seed dormancy release[68]. KAI2 can serve as an environmental sensor or integrator of environmental signals (light, temperature, water, and nutrients), to modulate seed dormancy via the balance of ABA and GA[69]. In seeds, KAR1 promotes seed dormancy release by increasing endogenous GA and repressing endogenous ABA (Fig. 3)[65,70].
-
Carbohydrates serve as energy substances for cell activities in plants and involve almost the whole plant life cycle including dormancy and growth. It includes soluble sugars (e.g., glucose, fructose, and sucrose) and non-soluble sugars like starch. Those carbohydrates can be transformed with each other through various biochemical metabolism. Finally, all carbohydrates can provide energy (ATP and NADH) for plants by glycolysis[71].
For geophytes, the dormant organs are sink organs and are enriched in carbohydrates. During bulb dormancy release by cold treatment, polysaccharides tend to be broken into oligosaccharides or monosaccharides that could be used to maintain the development and growth of plants[72−74]. In lily or tulip, starch is degraded into small molecular sugars and cold contributes to the increase of fructose, glucose, fucose, mannose, altrose, and so on which could be later largely used for bud development at the end of bulb dormancy[75−77]. In this process, several starch-degrading enzymes are involved, e.g., α-amylase, isoamylase, β-amylase, and α-glucosidase[78]. Moreover, in geophytes, accumulated sugars have to be transported by sugar transporters and PD, and then downloads to buds where sugars could be used as energy resources for cell division and elongation[79]. Endogenous ABA could repress the mobility and content of soluble sugars while GA plays the opposite role in geophyte dormant buds[24,48,79]. Although multiple research has shown the correlation between GDR and sugars, there is yet no genetic evidence.
Sucrose not only supports energy to plant development but also integrates plant development by acting as a signal molecular[24]. Sucrose is hydrolyzed into fructose, glucose, and uridine diphosphate glucose by invertases (INV) and sucrose synthase (SUS), respectively, and further phosphorylated by hexokinase (HXK1) to generate glucose-6-phosphate (Glc6P), resulting in trehalose-6-phosphate (T6P) and Glc1P[80]. T6P and Glc1P have conserved functions among plants that are involved in energy supply and signal transduction[80]. Sugar metabolic and signaling pathways integrate endogenous phytohormone signals as well as environmental signals (light, temperature, water, and nutrients) and further regulate or balance plant development[24,80]. HXK1 and REGULATOR OF G-PROTEIN SIGNALING 1 (RGS1) directly sense glucose, and SUS is a potential sucrose sensor[80]. In addition, Sucrose Non-Fermenting Related Protein Kinase 1 (SnRK1) and Target of Rapamycin (TOR) can sense energy status (Fig. 3)[24]. TOR is a growth activator and promotes mRNA translation and cell division by phosphorylating the mTOR Substrate S6 Kinase 1 (S6K1) when in a high energy state (e.g., high T6P, G1P, and G6P)[24]. But when in a low energy state, SnRK1 is active and can inhibit the TOR's activity by direct phosphorylation[24]. Modifying the transcriptional levels of SnRK1 and T6P in potatoes significantly affects the tuber's dormancy trait[81].
-
Epigenetics refers to the reversible and heritable changes in gene function without changing the DNA sequence. In plants, the main epigenetic regulation mechanisms include DNA methylation, histone modification, chromatin remodeling, and microRNAs (miRNAs)[82]. Although several reviews suggested that epigenetic regulations are involved in GDR, limited genetic evidence supports these postulates due to lack of efficient genetic transformations for GDR[24,82].
Histone modification
-
Histone modification is one of the most important epigenetic modifications in plants, which fine-tunes gene expression during plant development transitions by changing chromatin structure, and mainly includes acetylation, methylation, and ubiquitylation (Table 1)[24]. During DR in lily bulbs, genes related to histone modifications are changed dramatically, a similar tendency occurs in bud and seed dormancy[24,83,84]. During endodormancy release in Pyrus pyrifolia, H3K4me3 (H3 lysine 4 trimethylation) level at DAM locus is reduced[85]. Meanwhile, H3K27me3 is remarkably enriched at DAM loci[86]. In poplar, a negative regulator of bud dormancy, EEB3 (EARLY BUD BREAK3) is repressed in its transcript level by H3K27me3 when buds are in dormant states, and further extends the cell cycles in the shoot meristem[87]. Histone acetylation is also involved in GDR. In potato tubers, histone acetylation levels of histone H4, H3.1/3.2 are increased when GDR[88]. As it is well known that genes in pathways of flowering, vernalization, and ABA are proven to be involved in GDR, here, we summarize some histone modifications at these loci in Arabidopsis (Table 1). It is clear that GDR is regulated by histone modification but the mechanism remains unknown in geophytes.
Table 1. Dormancy associated genes regulated by epigenetics.
Modification type Regulation factors* Functional class* Target genes* References Chromatin remodeling complexes BRM SWI2/SNF2-like FT, CO, SOC1 [91] Chromatin remodeling complexes EBS BAH and PHD domain-containing protein FT [93] Heterochromatin LHP1 HP1 homologue FT [91] Histone deacetylation FLD Homologous to a subunit of histone deacetylase complexes FLC [91] Histone deacetylation FVE Putative subunit of histone deacetylase complex FLC [91] Histone monoubiquitination HUB1/2 E3 homologs FLC [94] Histone methylation MRG1/2 H3K36me3 FT [95] Histone methylation VRN1/2 H3k27me3 FLC, FT [91,96] Histone demethylation JMJ30 Histone demethylase SnRK2.8 [97] DNA methylation MET1 Maintenance CpNpG methyltransferase FLC [91] * BAH: bromo-adjacent homology; BRM: BRAHMA; CO: CONSTANS; EBS: EARLY BOLTING IN SHORT DAYS; FLC: FLOWER LOCUS C; FLD: FLOWER LOCUS D; FT: FLOWER LOCUS T; FVE: FLOWER LOCUS VE; HUB: HISTONE MONO-UBIQUITINATION; JMJ30: JUMONJI C DOMAIN-CONTAINING PROTEIN 30; LHP1: LIKE HETEROCHROMATIN PROTEIN; MET1: METHYLTRANSFERASE 1; MRG: MORF RELATED GENE 1; PHD: Plant homeodomain; SnRK: SNF1-related protein kinase 1; SOC1: SUPPRESSOR OF OVEREXPRESSION OF CO 1; SWI2/SNF2: SWITCHING2/ SUCROSE NONFERMENTING; VRN: VERNALIZATION. DNA methylation
-
In plants, DNA methylation occurs at cytosines in all sequence content (CG, CHH, and CHG)[89]. DNA METHYLTRANSFERASE 1 (MET1), CHROMOMETHYLTRANSFERASE2 (CMT2), and CMT3 are responsible for the maintenance of CG, CHH, and CHG methylation, respectively[89]. Low-temperature-induced GDR in lily bulbs and DNA demethylation is increased, which contributes to the cell division in shoot meristems[90]. In Arabidopsis, DNA methylation could regulate the vernalization gene (FLC) which is tightly controlled during the transition stage (Table 1)[91,92]. Currently, research about DNA methylation in GDR is less illustrated.
miRNA
-
MicroRNA (miRNA) is a special class of small RNAs (sRNAs) with a length of about 19 to 24 nucleotides in length which guide the post-transcriptional silencing of target genes with high complement to the miRNA[98]. In plants, miRNAs are involved in all aspects of plant development and transition stages from growth to dormancy, cell proliferation to differentiation, and vegetative to reproductive growth[99]. Besides, hormone signaling and environmental signals could be mediated by miRNAs, e.g., miRNA390 and miRNA319 mediate auxin signaling and low-temperature signaling by targeting ARF2 (AUXIN RESPONSE FACTOR2) and TCP1, respectively[99,100]. It is well known that miRNA-target gene modules are conserved, such as miR156/157-SPL, miR160-ARF, miR172-AP2, miR319-TCP, and miR390-TAS3 (ta-siRNAs act on ARF)[98]. But some conserved miRNAs also have gained unique targets in different species throughout the evaluation process, like miRNA396 in moss and miRNA390 in Physcomitrella patens[98].
Although much process has been achieved on perennial vegetative bud dormancy by RNA sequencing techniques in recent years, the genetic evidence is still missing (Table 2). In Japanese apricot (Prunus mume Sieb. et Zucc.), miR169 regulates the NF-Y complex to activate the bud dormant release[101]. In Lilium pumilum, several miRNAs were identified to be potentially involved in bulb dormancy, e.g., miR159, miR160, mi166, miR168 and miR396[90]. In apple, miR159 represses the transcriptional level of MdMYB33 and MdMYB65 and mediates bud dormancy release by balancing endogenous ABA homeostasis[102]. Other dormancy-associated genes, like DAM and CDPK1, are also reported to be regulated by miR6390 and miR390, respectively[103,104].
Table 2. miRNA related to bud dormancy.
miRNA Targets* Species Function Reference miR156 SPL Paeonia suffruticosa Bud dormancy release [105] miR159 MdMYB33 and MdMYB65 Malus domestica ABA homeostasis [102] miR159 MYB/TCP Lilium pumilum Bulb dormancy release [90] miR160 ARF Lilium pumilum Bulb dormancy release [90] miR169 HAP2 Populus tremuloides Vegetative bud dormancy [106] miR169 NF-YA Prunus mume Bud dormancy release [101] miR172 AP2 Paeonia suffruticosa Bud dormancy release [105] miR319c TCP2 Camellia sinensis Apical bud burst [107] miR390 TAS3 Pyrus pyrifolia Endodormancy release [108] miR390 CDPK1 Solanum tuberosum Tuber dormancy [104] miR6390 DAM Pyrus pyrifolia Dormancy
transition[103] * AP: APETALA; ARF: AUXIN RESPONSE FACTOR; CDPK: CALCIUM-DEPENDENT PROTEIN KINASE; DAM: DORMANCY-ASSOCIATED MADS-BOX; HAP: HAPLESS; MYB: MYELOBLASTOSIS; NF-YA: Nuclear Transcription Factor Y Subunit Alpha; SPL: SQUAMOSA PROMOTER BINDING PROTEIN-LIKE; TAS: TRANS-ACTING SIRNA3; TCP: TEOSINTE BRANCHED1/CYCLOIDEA/PCF. -
Nitric oxide (NO) is reported to be involved in various abiotic and biotic stress and plant physiology[109,110]. In potatoes, NO mediates tuber dormancy release and sprouting by regulating ABA metabolism that exogenous NO dramatically stimulates the expression of StCYP707A1 and inhibits the expression of StNCED1[111]. Although NO is accumulated in buds during dormancy release in grapevine and peach, the role of NO in GDR requires further investigation[112,113].
Bromoethane
-
Similar to NO, bromoethane accelerates tuber dormancy release in potato[114]. After bromoethane treatments, ABA catabolic genes (StCYP707A1, StCYP707A2 and StCYP707A3) were up-regulated while ABA biosynthesis genes (StNCED1, StNCED2 and ZEP family genes) were down-regulated, and resulted in a low ABA content that accelerated dormancy release[115].
Glycerol
-
In some geophytes, glycerol is enriched in dormant storage organs, such as Easter lily and yam[75,116]. During GDR in lily, glycerol content is decreased in scales along with dramatic changes in glycerol-related genes. Moreover, exogenous glycerol treatment significantly enhances NCED expression and delays dormancy release as well as flower transition[75]. However, the effect of glycerol on dormancy release in other geophytes is less documented.
-
Wounding treatments are stress signals which can stimulate a series of complex physiological and biochemical reactions in plants, regulating DNA synthesis, respiration rates and wound-induced hormones (e.g., ethylene and ABA)[117]. Wounding treatments by cutting, pruning, stabbing, etc, have been found to be practical ways to promote the dormancy release in geophytes (onion and potato), vine buds (grapevine), and seeds (Arabidopsis)[113,118−120].
The wounding treatments significantly promote GDR by increasing cell respiration in dormant organs. The wounding respiration is in collaboration with wound healing reactions and promotes callus formation, lignin, and xanthan[121]. Accompanied by carbohydrate catabolism, respiration activates glycolysis and pentose phosphate pathways and provides energy for dormancy release[122]. In potatoes, respiration stimulates starch glycolysis and leads to faster tuberous sprouting and thick sprouts[122,123].
Due to the wounding treatments, phytohormone levels (like ethylene and ABA) are changed which further affects the speed of dormancy release. Wound-induced ethylene is well documented and that the ethylene precursor ACC is accumulated in injured tissues[124]. Elevated ethylene could regulate GDR as described above. Wounding treatments can decrease endogenous ABA in potato tubers[125]. A similar observation occurs in dormant corms in Gladiolus where cutting treatment also represses the expression of NCED and ABA content. But regulating networks between the wounding signal and ABA/ ethylene are still unclear.
Wounding treatments also increase the capacity of dormant tissues to meet with oxygen and water and cause early sprouting. These methods are technically useful for seeds with compact seed coats or pericarp-testa like lotus and celery seeds. Heme-binding proteins can function as sensors for oxygen and nitric oxide, which can directly repress the activity of DOG1 (DELAY OF GERMINATION) protein and further release seed dormancy[89]. Once water uptake has occurred, the water potential thresholds for radical were changed, resulting in germination[126]. The detailed mechanism of wounding-induced seed germination is well documented but is not the main focus of the current review.
-
In nature, plants have to make changes to adapt to harsh environmental conditions, and varieties of dormancy have evolved for different organs, like seed dormancy, bud dormancy, and geophyte dormancy. Here, we mainly summarize recent progress on how geophytes sense and respond to environmental factors (temperature and light) during GDR, the effects of endogenous hormones, carbohydrates, and epigenetics on GDR, and the mechanism of some small molecular and wounding treatments on GDR.
Despite our growing knowledge of seed dormancy in model plants, e.g., Arabidopsis, rice, and wheat, many secrets remain to be decoded in the field of geophytes. Currently, we cannot find an accurate physiological symbol to divide the dormancy and the start point of dormancy release. In lily, it is suggested that the inflection point of soluble sugars refers to the start point of dormancy release[78]. However, we lack the necessary evidence and has not been proven in other geophytes. Due to large genome sizes (~1 Gb to 50 Gb), long juvenile periods, and low genetic transformant efficiencies for geophytes, the regulating network for GDR is largely unclear. Compared with seed dormancy, developing gene markers of the dormant trait in geophytes is lacking. With the rapid advance of high-throughput omics sequencing (genomics, proteomics, metabolomics, ChIP-seq, and others) and achievements of transgenic strategy in geophytes, it will generate a broad and detailed picture of geophyte dormancy which contributes to distinguishing geophyte dormancy with other types of dormancy on the aspect of plant evolution, response to environmental factors, changes in specific cells (metabolism, epigenetics, transcripts) and molecular breeding for new cultivars of geophytes with various degrees of dormancy.
We apologize for not citing many references due to space limitations. We thank Dr. Hongzhi Wu (Yunnan Agricultural University) for supporting the picture of Zantedeschia hybrida Spr. This work was funded by Beijing Natural Science Foundation (6212012 to J.W.), National Natural Science Foundation projects (grants 3217180532; 31701952 to J.W. and 31902047 to J.J.S.), Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF- PXM2019_014207_000032), Natural Science Foundation of Anhui Province (2008085MC79 to J.J.S.), The 2115 Talent Development Program of China Agricultural University and 111 Project of the Ministry of Education (B17043).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Yajie Zhao, Chang Liu, Juanjuan Sui
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao Y, Liu C, Sui J, Liang J, Ge J, et al. 2022. A wake-up call: signaling in regulating ornamental geophytes dormancy. Ornamental Plant Research 2:8 doi: 10.48130/OPR-2022-0008
A wake-up call: signaling in regulating ornamental geophytes dormancy
- Received: 22 March 2022
- Accepted: 14 May 2022
- Published online: 30 May 2022
Abstract: Ornamental geophytes are a group of important flowers worldwide. As perennial plants, geophytes go through several rounds of life cycle under seasonal climates. The dormant trait of underground modified organs in geophytes is critical for the process of storage, planting as well as breeding. Although the dormant physiology in geophytes is complex and largely unknown, several advancements have been achieved in this field. Here, we review the knowledge on the role of environmental factors, endogenous hormones, carbohydrates, and epigenetics in the regulation of geophytes dormancy release (GDR). We also discuss dormancy release (DR) methods and their roles in geophytes, including small molecular chemicals and wounding treatments.
-
Key words:
- Geophytes /
- Dormancy /
- Environmental factors /
- Hormones /
- Carbohydrates /
- Epigenetics












