-
Tobacco is a crop that is adversely affected by continuous cropping, leading to increased disease incidence, decreased yield, and reduced quality[1, 2]. Due to limitations in arable land area, tobacco-growing regions in Fujian Province (China) mainly employ a tobacco-rice rotation system. However, as the duration of rotation increases, the problems associated with continuous cropping become more severe, posing a significant challenge to the sustainable development of tobacco production[3]. Previous studies showed that long-term tobacco-rice rotation increased the secretion of autotoxins in tobacco roots, leading to the deterioration of soil's physical and chemical properties[4]. Additionally, the flooding required for rice cultivation decreases soil aeration, resulting in soil anaerobiosis[5]. These factors contribute to a decline in soil available nutrients[6, 7], ultimately decreasing crop yield and quality. Farmers often resort to increasing nitrogen fertilizer application when faced with declining yields. However, traditional nitrogen application rates for tobacco have far exceeded the nitrogen absorption capacity of the plants. Excess nitrogen residue in the soil leads to soil degradation, acidification, and decreased organic matter content, among other negative effects[8]. In the context of a rice-fallow rotation system, the relatively long periods of aerobic conditions in the soil and soil degradation reduce soil's ability to retain mineralized nitrogen. As a result, ammonium nitrogen is more readily nitrified, and nitrate nitrogen is more prone to leaching, accelerating soil acidification and nitrogen loss[9]. This further exacerbates the over-application of nitrogen fertilizer in tobacco-rice rotation systems, creating a vicious cycle. Therefore, finding a way to reduce nitrogen fertilizer usage, minimize nitrogen loss, and improve soil conditions while maintaining crop yield is a crucial issue that needs to be addressed for the sustainable development of agriculture.
Biochar, as a soil amendment rich in stable carbon, has significant theoretical and practical implications for understanding its impact on soil nitrogen cycling. In recent years, biochar has played an important role in soil improvement and increasing crop yield. It is a stable product obtained from the thermochemical conversion of biomass or organic waste under high-temperature anaerobic or oxygen-limited conditions[10]. The characteristics of biochar, such as high carbon content and recalcitrance, make it highly potential in soil carbon sequestration and reduction of greenhouse gas emissions[11, 12]. Previous research revealed that biochar utilization potentially influenced the growth and development of tobacco plants, including their chemical composition, sensory quality, and yield[13]. Moreover, biochar application has shown great potential in increasing soil organic carbon content, improving soil microbial environment, enhancing soil fertility, and increasing fertilizer use efficiency. He et al.[14] found that under nitrogen reduction conditions (180 kg∙ha−1), biochar application at a rate of 15 t∙ha−1 improved soil fertility and significantly increased crop yield. As research deepens, the microbial-mediated mechanisms underlying biochar's promotion of nutrient utilization in crops have gained traction among researchers and farmers. Microorganisms are essential and active components of soil ecosystems, and the absorption and utilization of soil nitrogen by crops rely on the dominant soil nitrogen cycling processes they mediate[15]. Zhang et al.[16] demonstrated that biochar remediation of tobacco soils in a tobacco-rice rotation system improved soil physical and chemical properties, thereby facilitating the transition of soil nitrogen cycling microorganisms to more efficient nitrogen utilization pathways. However, insufficient literature exists regarding the appropriate nitrogen reduction ratio for enhancing soil quality and maximizing crop nitrogen utilization following the application of biochar in a continuous tobacco-rice rotation field. This study sought to explore nitrogen absorption and utilization patterns of tobacco crops and the response characteristics of functional genes related to soil nitrogen cycling subjected to the interaction of reduced nitrogen utilization ratios following biochar application in a long-term tobacco-rice rotation field. Our findings will provide a scientific basis for the developing nitrogen reduction strategies that enhance the quality and efficiency of tobacco production.
-
The field trial was conducted at the Tobacco Agricultural Research Base in HuanxiTown, Jin'an District, Fuzhou City, Fujian Province, China (119°36′86″ E, 26°17′33″ N). The trial soil was a red paddy soil that had been continuously cropped with tobacco-rice rotation for 20 years. The trial was conducted for a consecutive period of 2 years, from January 2021 to August 2022. During the tobacco growing season in 2021, the accumulated temperature was 2,445.59 °C, and the effective accumulated temperature was 1,159.83 °C. The total rainfall was 459.11 mm. In 2022, the accumulated temperature during the tobacco growing season was 2,009.04 °C, with an effective accumulated temperature of 869.92 °C. The total rainfall was 610.43 mm. At the start of the trial, the initial soil pH was 4.95. Organic matter content was 28.69 g∙kg−1, total nitrogen content was 2.18 g∙kg−1, total phosphorus content was 0.60 g∙kg−1, total potassium content was 20.43 g∙kg−1, alkali-hydrolyzable nitrogen content was 94.00 mg∙kg−1, available phosphorus content was 40.96 mg∙kg−1, and available potassium content was 209.21 mg∙kg−1.
Experimental design
-
The experimental material used was the 'Yunyan 87' flue-cured tobacco commonly grown in Fujian (China). The biochar used in the experiment was obtained by high-temperature pyrolysis (around 450 °C) of tobacco stalks under anaerobic conditions provided by Sanli New Energy Co., Ltd. in Shangqiu, Henan Province (China). The physicochemical properties of the biochar were as follows: pH 9.66, fixed carbon content 475.90 g∙kg−1, total nitrogen content 15.00 g∙kg−1, total phosphorus content 1.40 g∙kg−1, and total potassium content 20.10 g∙kg−1. The experiment consisted of five treatments. The nitrogen application rates followed the local recommended doses, with the same biochar application rate (30 t∙ha−1). The treatments were as follows: T1: Conventional nitrogen application rate of 127.50 kg∙ha−1, T2: Nitrogen application rate reduced by 10% (114.75 kg∙ha−1), T3: Nitrogen application rate reduced by 20% (102.00 kg∙ha−1), T4: Nitrogen application rate reduced by 30% (89.25 kg∙ha−1), T0: Control treatment with no biochar and the conventional nitrogen application rate of 127.50 kg∙ha−1. The experiment was set up in a randomized block design with three replicates per treatment. Each plot covered an area of 144 m2 (24 m long, 6 m wide), resulting in a total of 15 plots. A plot with no biochar or nitrogen fertilizer (N0) was included to calculate nitrogen uptake efficiency. The phosphorus and potassium fertilizers were applied uniformly for all treatments, with P2O5 at a rate of 99 kg∙ha−1 and K2O at 402 kg∙ha−1. In December 2020, the biochar was evenly spread on the soil surface and incorporated into the soil by rotary tillage, with a ridge height of 35 cm and row spacing of 1.2 m × 0.5 m before planting the tobacco. Later, we applied the reduced nitrogen fertilizer in January 2021 and 2022 at different rates. Apart from the variations in treatments, the rest of the field management followed the production standards for high-quality tobacco leaves in Fujian (China). In 2022, the experiment continued with the reduced nitrogen application treatments, and qRT-PCR analysis of key nitrogen cycling functional genes in the tobacco rhizosphere soil was conducted.
Determination items and methods
Sampling and preparation of plant root, leaf, and stem
-
During the harvest period of flue-cured tobacco, three representative plants with uniform growth from each experimental treatment were selected. Various organs of these plants, including roots, stems, and leaves were sampled. The complete root system of the tobacco plants was excavated, and any debris around the root zone was quickly removed. The soil adhering to the roots was wrapped in aluminum foil, mixed, and frozen in liquid nitrogen before being transferred and stored at −80 °C in a freezer for further use. Each organ was then dried at 105 °C for half an hour to kill the tissues and further dried at 80 °C until a constant weight was reached. The dry matter weight of each sample was determined.
Determination of nitrogen accumulation in flue-cured tobacco
-
After grinding the samples using a 0.25 mm mesh, the total nitrogen content of the plant samples was measured using the concentrated sulfuric acid-hydrogen peroxide digestion method. The measurements were conducted using a fully automated chemical analyzer (Smartchem 2000) from Germany. Nitrogen accumulation and nitrogen use efficiency were calculated using the following formulas[17]:
Nitrogen accumulation per unit area of plant (kg·ha−1) = Nitrogen accumulation per plant × planting density
Nitrogen uptake efficiency (%) = [(Nitrogen uptake by plants in nitrogen treatment area − Nitrogen uptake by plants in the area without nitrogen treatment) / Nitrogen application rate] × 100%
Nitrogen harvest index = Nitrogen accumulation in plant leaves / Whole plant nitrogen accumulation
Determination of nitrogen content of different forms in flue-cured tobacco cultivated soils
-
During the root extending, vigorous growing, and harvest periods of flue-cured tobacco, a five-point sampling method was used to collect five soil cores from each plot. Before mixing the soil samples from the same layer, a single sample was provided from each replicate plot. Three replicates were taken from each treatment. A total of 45 soil samples were collected from the 0−30 cm soil layer (3 × 5 × 3). The samples were air-dried indoors and ground using a 1 mm mesh. The total nitrogen content was determined using the concentrated sulfuric acid digestion method with a fully automated chemical analyzer (Smartchem 2000)[17]. Nitrate nitrogen was measured using the dual-wavelength spectrophotometric colorimetric method[18], ammonium nitrogen was determined using the indophenol blue colorimetric method[19], and soil alkali-hydrolyzable nitrogen was measured using the alkaline hydrolysis diffusion method[20].
Determination of physicochemical properties in flue-cured tobacco cultivated soils
-
During the harvest period of flue-cured tobacco, the sampling method was the same as described above. The pH was determined using the potentiometric method with an acidity meter[21]. The soil organic matter content was determined using the potassium dichromate titration method[22]. Additionally, three soil ring samples were taken from each replicate point. Soil was extracted from the primary root of the tobacco plant at a depth ranging from 30 to 40 cm below the root's base. The cut ring samples were immediately covered to prevent moisture evaporation and then weighed and recorded to determine the soil bulk density, porosity, and field moisture capacity[23].
qRT-PCR determination of key nitrogen cycle functional genes in flue-cured tobacco rhizosphere soil
-
During the vigorous growing period of tobacco, three representative tobacco plants with uniform growth were selected for each experimental treatment. The complete root system of the tobacco plants was excavated, and the surrounding debris was quickly removed from the rhizosphere. The soil adhering to the roots was wrapped with aluminum foil, and the soil samples were mixed and placed into liquid nitrogen for freezing. They were then transferred and stored in a −80 °C freezer for later use. A fresh soil sample weighing 0.25 g was taken, and soil genomic DNA was extracted using a soil genomic DNA extraction kit (DcP336, TianGen Biotech Co., Ltd., Beijing, China). After DNA concentration detection, qRT-PCR validation was performed on key functional genes associated with soil nitrogen cycling. The specific primer sequences can be found in Supplemental Table S1. Amplification was carried out using a fluorescence quantitative PCR instrument (ABI7500, Applied Biosystems, USA). The qPCR reaction system consisted of 20 μL, including 10 μL 2× Taq MasterMix, 7 μL sterile water, 0.5 μL of each upstream and downstream primer, and 2 μL of template DNA. The standard curve was constructed using the Ct value as the abscissa and the log value of the copy number of the standard sample as the ordinate (log10). The PCR reaction was conducted with the following program: 95 °C for 30 s, followed by 40 PCR cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 40 s. To establish the melting curve of the PCR product, after the amplification reaction, the program consisted of 95 °C for 10 s, 60 °C for 60 s, and then gradually heated to 99 °C from 60 °C (with a ramp rate of 0.05 °C/second).
Data processing and statistical analysis
-
Tables and figures were plotted with Microsoft Excel 2019, Origin 2022b, R4.2.2 software, and significant difference among the different treatments were tested using SPSS 22.0 statistical software (Duncan's multiple range tests, α = 0.05). Linear regression analysis, stepwise regression analysis, and random forest model prediction analysis with Mantel detection using R4.2.2.
-
According to Table 1, the plant nitrogen accumulation showed significant differences among treatments and years, as well as significant interaction effects between treatments and years. Nitrogen accumulation in the tobacco leaves showed significant differences among treatments, but no significant differences were observed between years and the interaction of years and treatments. In 2021, the leaf and plant nitrogen accumulation in the biochar treatments (T1−T4) was significantly higher than that in the T0 treatment, ranging from 21.45% to 64.95% and 13.91% to 48.88%, respectively. Regarding plant nitrogen accumulation, the rankings among the treatments for both years were T1 > T2 > T3 > T4, with T0 being the lowest. However, in terms of leaf nitrogen accumulation, there was no significant difference between T1 and T2 in a two-year comparison, while T1 and T3 in 2021 revealed the opposite, but no significant difference was observed in 2022.
Table 1. Effect of reduced nitrogen fertilizer application combined with biochar on nitrogen accumulation and utilization in flue-cured tobacco.
Year Treatment Leaf (kg·ha−1) Stem (kg·ha−1) Root (kg·ha−1) Whole plant (kg·ha−1) Nitrogen uptake efficiency (%) Nitrogen harvest index 2021 N0 20.98d 9.76d 9.12c 39.86f − − T0 34.56c 18.46c 21.04b 74.07e 26.83c 0.47c T1 55.80a 25.60a 29.76a 111.16a 55.93a 0.50b T2 56.17a 24.76a 23.18ab 104.06b 55.95a 0.54a T3 49.03b 24.31a 22.39b 95.73c 54.78a 0.51ab T4 42.80b 21.48b 20.78b 85.06d 50.64b 0.50b 2022 N0 19.21d 5.99c 6.04e 31.23e T0 32.68c 19.15ab 17.43c 69.26d 29.83d 0.47c T1 48.32a 23.20a 22.09a 93.61a 49.71a 0.52b T2 46.79a 20.35b 19.20b 86.33b 48.02a 0.54a T3 44.87a 18.66ab 17.56bc 81.10c 48.89a 0.55a T4 36.29b 18.10b 15.11d 69.49d 42.87c 0.52b Year(Y) ** ** ** ** ** ** Treatment (T) ** ** ** ** ** ** Y × T NS ** NS ** ** NS Note: Nitrogen uptake efficiency for the T0, T1, T2, T3, and T4 treatments was calculated using N0 as the control. Different lowercase letters in the same column of the table indicate the difference between treatments at 0.05 level in the same year (p < 0.05), the same was applied in Table 2 . '*' indicates a significant difference at 0.05 level; '**' indicates a highly significant difference at 0.01 level; NS indicates no significant differences at 0.05 level. Nitrogen accumulation in leaves includes nitrogen in the topped leaves. The nitrogen uptake efficiency of tobacco plants in the biochar treatments was significantly higher than that of the T0 treatment during the two-year period, with an increase of 23.81% to 29.12% in 2021, reaching as high as 55.95%. In 2022, the increase in nitrogen uptake efficiency decreased to a range of 13.04% to 19.88%, with the most significant decrease observed in the T4 treatment. There was no significant difference in nitrogen uptake efficiency between the T2 and T3 treatments among the nitrogen reduction treatments during the two-year period. Further analysis of the nitrogen harvest index of tobacco plants showed that the biochar treatments had significantly higher values than the T0 treatment during the two-year period, with increases ranging from 7.79% to 15.83% and 10.64% to 17.02%, respectively. There was no significant difference between the T2 and T3 treatments in terms of nitrogen harvest index. In 2022, the T2 and T3 treatments were significantly higher than the T1 treatment, accounting for 3.85% and 5.78%, respectively.
Effect of reduced nitrogen fertilizer application combined with biochar on nitrogen content of different forms and physicochemical properties in flue-cured tobacco cultivated soils
-
As shown in Fig. 1, the highest values of alkali-hydrolyzable nitrogen, nitrate nitrogen, and ammonium nitrogen in the soil occur during the vigorous growing period. Over the course of two years, the biochar treatments significantly increased the nitrate nitrogen content in the soil during the vigorous growing period while significantly reducing the ammonium nitrogen content. The different forms of nitrogen in the soil exhibited a significant decrease with reduced nitrogen application rates. Furthermore, the biochar treatments significantly reduced the ammonium nitrogen content in the soil during the harvest period, and the alkali-hydrolyzable nitrogen content in the soil decreased significantly with reduced nitrogen application rates.
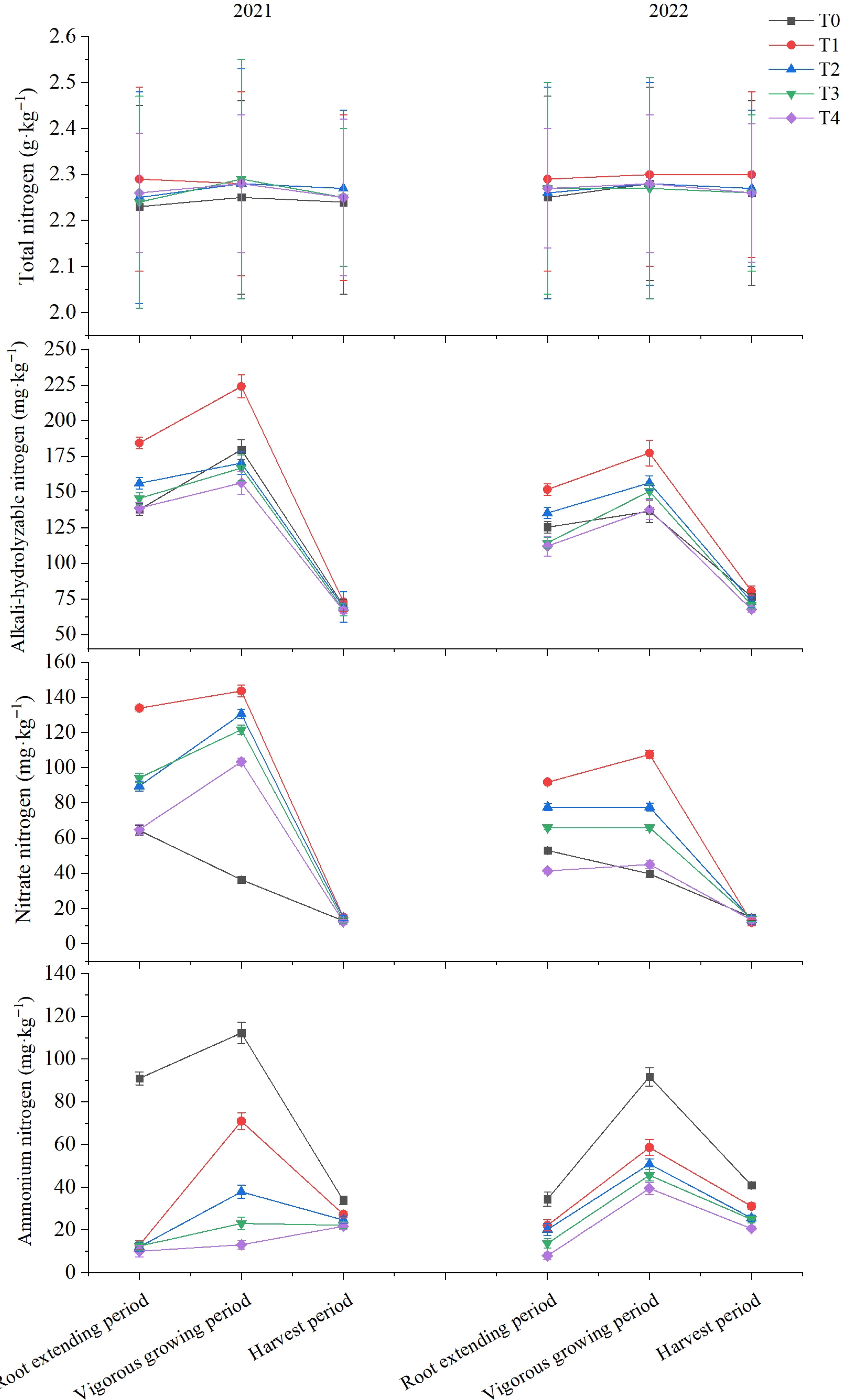
Figure 1.
Effect of reduced nitrogen fertilizer application combined with biochar on nitrogen content of different forms in flue-cured tobacco cultivated soils.
Table 2 revealed that during the two-year period, the biochar treatments significantly increased soil pH, organic matter content, porosity, and field water-holding capacity compared to the T0 treatment. Besides, these treatments significantly reduced soil bulk density, implying that biochar amendment is crucial in regulating soil pH values, organic matter content, soil porosity, and moisture capacity in acidic soil.
Table 2. Effect of reduced nitrogen fertilizer application combined with biochar on physicochemical properties in flue-cured tobacco cultivated soils.
Year Treatment pH Soil organic matter
(g·kg−1)Bulk density
(g·cm−3)Porosity
(%)Field moisture capacity
(%)2021 T0 4.96c 29.07b 1.34a 49.44b 33.70b T1 5.16a 31.98a 1.14b 57.19a 49.00a T2 5.18a 31.32a 1.12b 57.99a 47.33a T3 5.16a 31.48a 1.13b 57.34a 46.89a T4 5.15a 31.40a 1.12b 57.74a 44.23a 2022 T0 4.98c 28.89b 1.37a 48.43b 34.57b T1 5.15a 31.76a 1.18b 55.45a 47.00a T2 5.14a 31.93a 1.17b 55.98a 47.66a T3 5.17a 31.41a 1.14b 56.79a 46.56a T4 5.17a 31.15a 1.13b 57.22a 43.90a Difference of nitrogen cycle function gene abundance in rhizosphere soil
-
The treatment with biochar significantly increased the abundance of the ammonia oxidation gene AOA-amoA and denitrification gene narG compared to the T0 treatment, with an improvement range of 14.72% to 19.63% and 26.37% to 41.76%, respectively. The abundance of the denitrification gene norB was significantly lower than the T0 treatment, with a decreased range of 33.14% to 80.63%. As the nitrogen application decreased, the abundance of nitrogen cycling functional genes showed varying degrees of decrease between the denitrification treatments. However, the T2 and T3 treatments still significantly increased the abundance of the nitrification, nitrogen fixation, ammoniation, organic nitrogen synthesis genes, including nxrA, nifH, UreC, and gdh, with improvement ranges of 96.31% to 166.81%, 17.60% to 131.71%, 16.88% to 118.18%, and 24.04% to 74.22%, respectively (Fig. 2 & Supplemental Table S2).
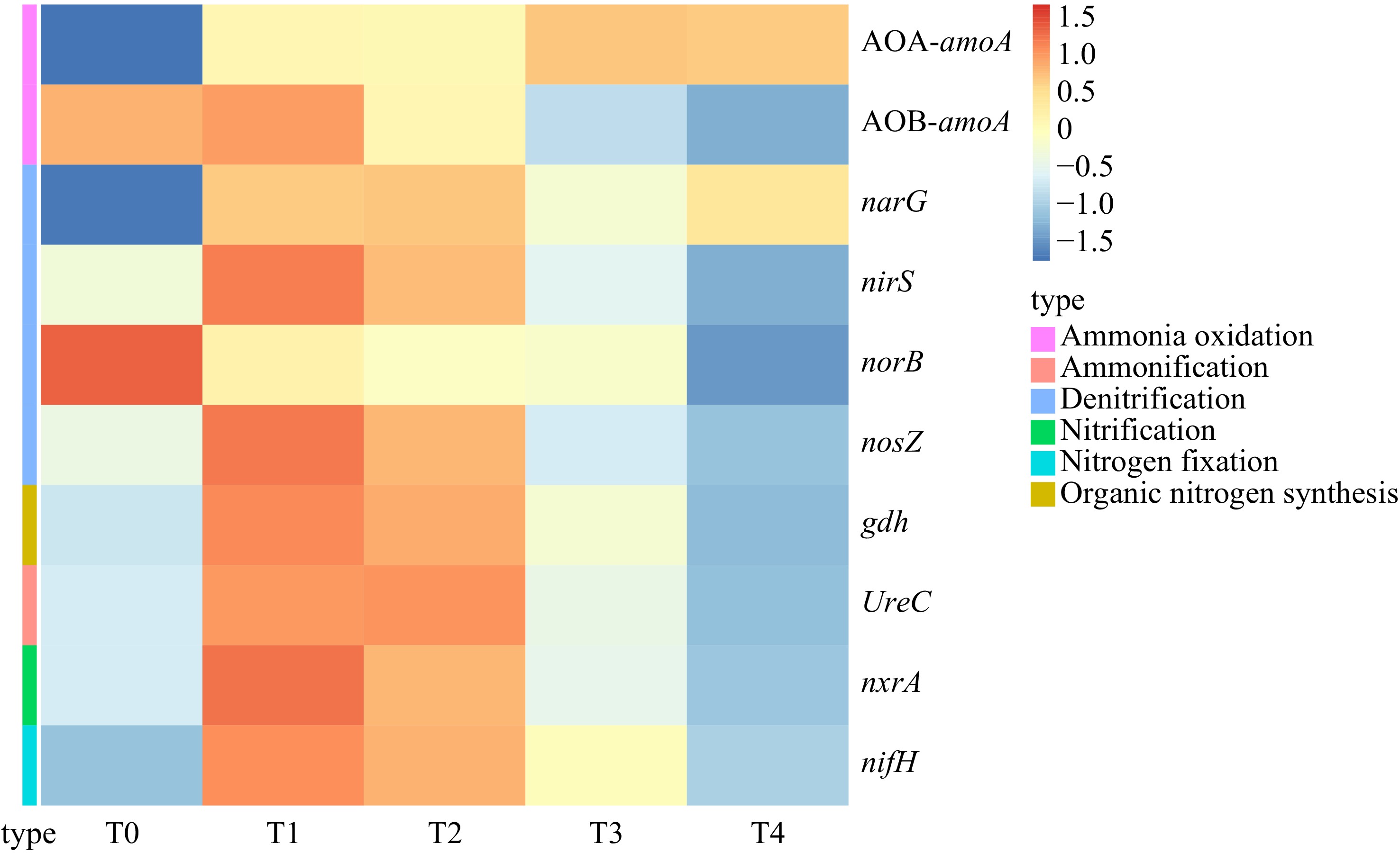
Figure 2.
Difference of nitrogen cycle function gene abundance among different treatments and control.
Effects of nitrogen cycle functional gene abundance on nitrogen metabolism characteristics of flue-cured tobacco
-
Random forest model analysis results (Fig. 3) showed that nitrogen cycling functional genes have a significant impact on the nitrogen uptake efficiency of tobacco, with a total explanatory rate of 90.08% (p < 0.001). Among them, nitrogen fixation, organic nitrogen synthesis, ammonification, and denitrification genes, including nifH, gdh, UreC, and nirS were significantly correlated (p < 0.01) with nitrogen uptake efficiency, respectively. The nitrification and denitrification genes, including nxrA and nosZ were significantly correlated (p < 0.05) with nitrogen uptake efficiency, respectively.
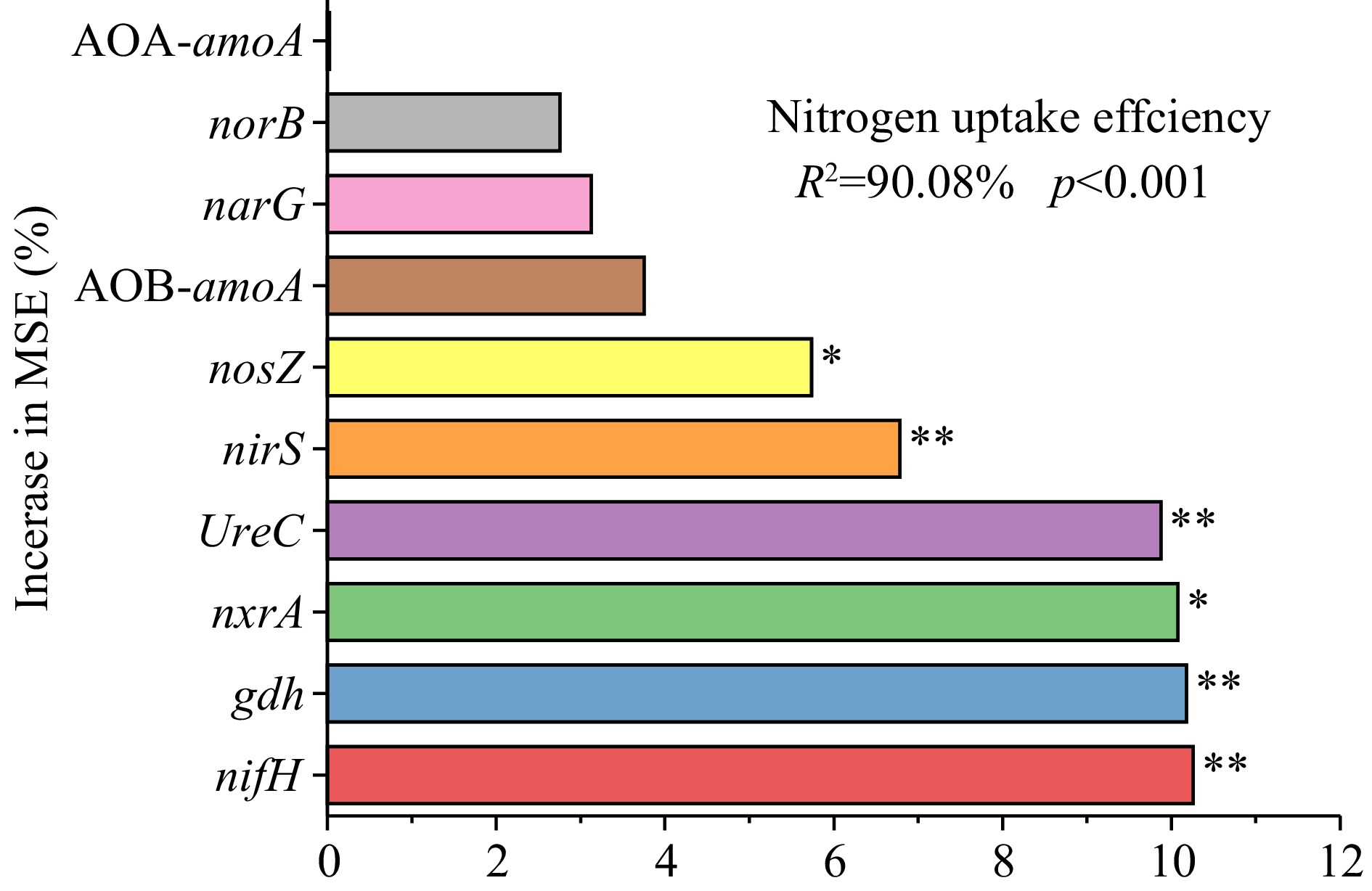
Figure 3.
Random forest model predicts the relevance of nitrogen cycle functional genes on nitrogen uptake efficiency in flue-cured tobacco. Note: The precision importance measures were calculated for each tree in a random forest and averaged over the entire forest. The percentage increase in the mean squared error (MSE) of the variables was used to estimate the importance of these predictors.
Regression analysis was conducted on tobacco plants' nitrogen accumulation and nitrogen uptake efficiency in 2021 and 2022 with different forms of nitrogen content during the vigorous growing period, as shown in Fig. 4. The nitrogen accumulation and nitrogen uptake efficiency of the whole plant showed a linear growth relationship with the nitrate nitrogen content, revealing slopes of 0.346 and 0.229, respectively (p < 0.001). The plant nitrogen accumulation also showed a stable, increasing trend with the alkali-hydrolyzable nitrogen content, exhibiting a slope of 0.43 (p < 0.01). Whereas the ammonium nitrogen content showed a negative growth trend with the nitrogen uptake efficiency (p < 0.01).
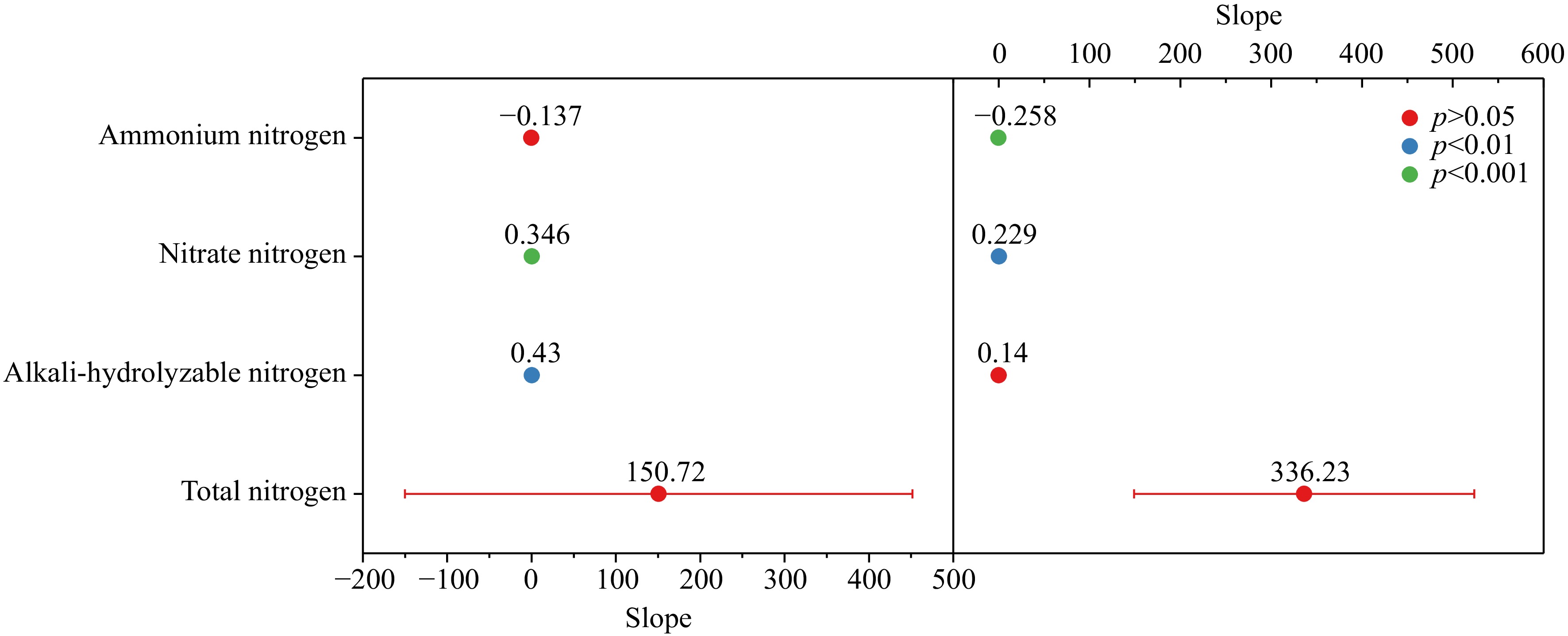
Figure 4.
Regression analysis on different forms of nitrogen content and nitrogen accumulation, absorption and utilization efficiency in soil.
The Mantel test results showed a highly significant positive correlation (p < 0.001) between the alkali-hydrolyzable nitrogen and nitrate nitrogen content and the abundance of UreC, nxrA, and nifH. Additionally, there was a significant negative correlation between the nitrate nitrogen content and the abundance of the norB gene (p < 0.05). A highly significant positive correlation was also observed (p < 0.001) between the ammonium nitrogen content and the abundance of the norB and AOB-amoA, while there was a significant negative correlation (p < 0.001) between the ammonium nitrogen content and the abundance of the AOA-amoA (Fig. 5).
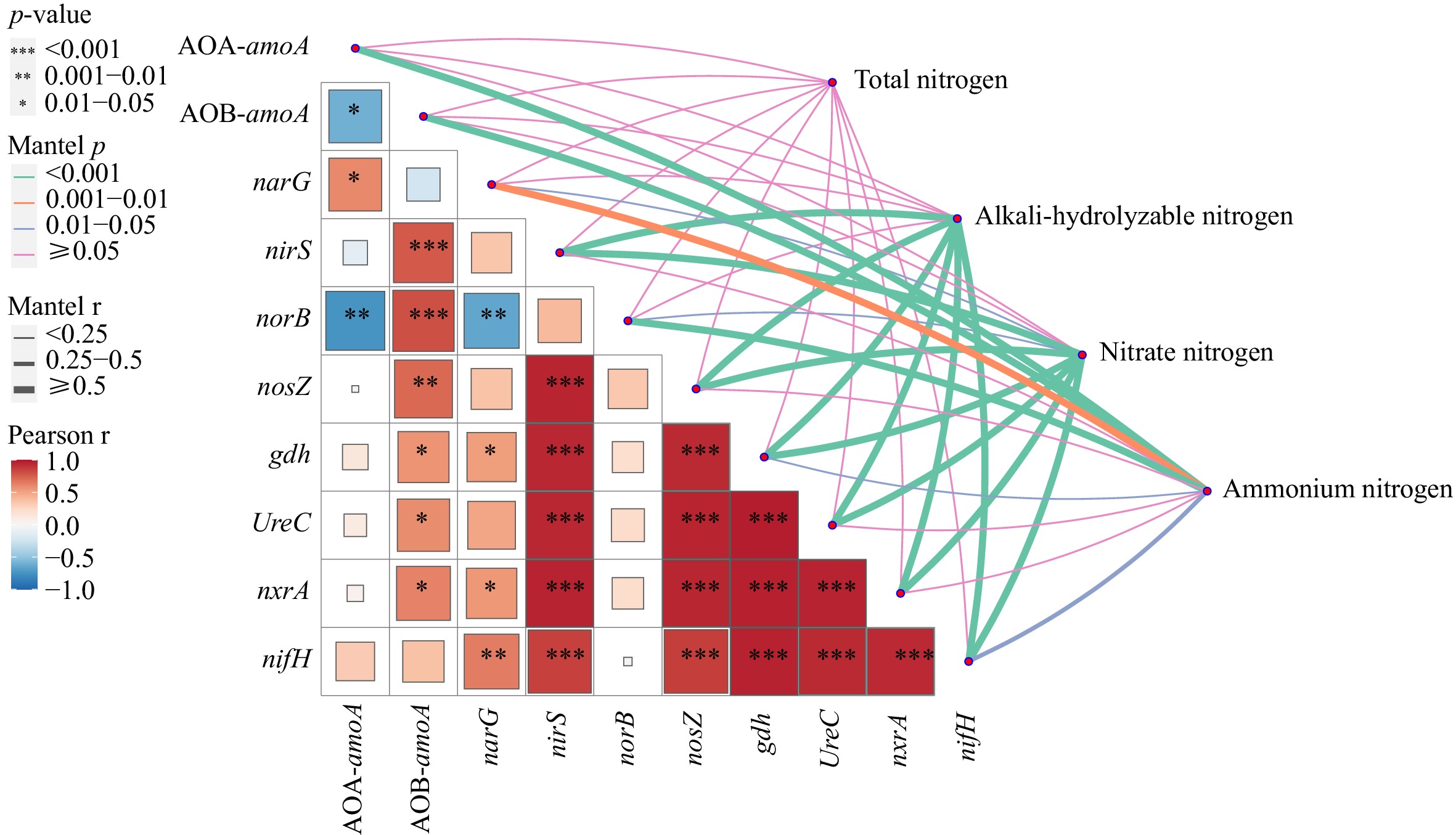
Figure 5.
Mantel test of different forms of nitrogen content and nitrogen cycle function gene abundance.
Further stepwise multiple regression analysis indicates that organic matter was the main factor influencing the abundance of most nitrogen cycling functional genes (AOB-amoA, gdh, nxrA, and nifH) in the rhizosphere soil (Table 3). Additionally, pH was an important soil physicochemical property influencing the ammonia oxidation gene AOA-amoA and denitrification gene norB. Whereas soil bulk density and porosity were the main factors influencing the nitrification gene nxrA.
Table 3. Soil physicochemical factors significantly related to the abundance of nitrogen cycle functional genes (screened by stepwise multiple regression).
Dependent variable Explanatory variable R2 P-value AOA-amoA Bulk density, pH 0.78 <0.01 AOB-amoA Organic matter 0.75 <0.01 narG pH, field moisture capacity 0.85 <0.01 nirS NA norB pH 0.68 <0.01 nosZ NA gdh Organic matter 0.41 <0.05 UreC NA nxrA Organic matter, bulk density, porosity 0.56 <0.01 nifH Organic matter 0.47 <0.01 Only explanatory variables with p < 0.05 are shown in the table. NA represents no best fit model, and R2 represents the proportion of variance explained by the model. -
Biochar is widely used in various fields such as soil improvement and nitrogen reduction to enhance efficiency due to its excellent properties[24]. Ge et al.[25] showed that the application of biochar increased nitrogen uptake efficiency in tobacco plants even with a 15% reduction in nitrogen fertilizer. Furthermore, continuous biochar application has shown significant effects on nitrogen retention and improves the utilization efficiency of tobacco plants. We found that treating soil with a history of continuous tobacco-rice rotation (20 years) with reduced nitrogen and biochar application significantly increased the nitrogen accumulation and uptake efficiency of tobacco plants in the first year compared to the control treatment. This behavior aligns with the work conducted by Li et al.[26], wherein they revealed that maize nitrogen uptake and nitrogen content were largely driven by the addition of nitrogen fertilizer and biochar, triggering higher dry matter accumulation, leaf photosynthetic efficiency, and grain yields. Our finding also agrees with a previous study, demonstrating that the combined use of nitrogen and biochar fertilizer enhanced the effectiveness of biochar in improving nitrogen use efficiency in plants[27]. This suggests that combining nitrogen fertilizer with biochar promotes plants' assimilation and adsorption of nitrogen. It could serve as a nitrogen source, providing essential plant development and growth substrates[28]. This also indicates that appropriate reduction of nitrogen coupled with biochar application enhanced nitrogen absorption capacity in tobacco plants, with a certain degree of persistence[29]. Therefore, short-term moderate nitrogen reduction may not decrease the nitrogen accumulation in crops. Cheng et al.[30] found that applying biochar significantly increased the nitrogen allocation rate in crop harvest organs. We observed that the increase in nitrogen accumulation in tobacco is primarily due to an increase in nitrogen accumulation in the leaves. As nitrogen application decreases, the overall nitrogen accumulation in the tobacco plant decreases to varying degrees. However, in terms of nitrogen accumulation in the leaves, there is no significant difference between the T2, T3, and the T1 treatment. Additionally, these treatments significantly increased the nitrogen harvest index. Previous studies have highlighted the issue of 'source-sink imbalance' after biochar application, which affects the transport of nitrogen nutrients to crop harvest organs[31]. It can be observed that under low nitrogen fertilizer treatment, nitrogen in tobacco plants was supplied to the leaves of the plant and will not lead to excessive nitrogen retention in the root and stem organs, thus reducing nitrogen loss caused by agricultural waste.
Previous studies have shown that during the vigorous growing period, tobacco plants rapidly increase their nitrogen uptake[32]. Applying biochar can significantly reduce the leaching of nitrate nitrogen in tobacco soil through its adsorption capacity[33]. We noticed that the inorganic nitrogen content reached its highest value during the vigorous growing period, and the biochar treatments significantly increased the nitrate nitrogen content and reduced the ammonium nitrogen content in the soil during this period. This behavior aligns with the work conducted by Han et al.[34], wherein they revealed that the nitrate nitrogen increased with biochar application and biochar application has little influence on the soil ammonium nitrogen. We believed this finding was associated with the enhancement of soil nitrification triggered by the application of biochar. However, with the decrease in nitrogen application rate, the difference in mineral nitrogen content between the treatment with 10% to 20% nitrogen reduction and biochar application and the control treatment was not significant. This suggests that moderate nitrogen reduction combined with biochar application is beneficial for improving soil fertility, which is similar to the findings reported by Sarfraz et al.[35]. This is mainly due to the fact that biochar itself provides nutrients and retains them, and also alters the dynamics of soil microorganisms, thus promoting biological carbon fixation to enhance soil fertility[36].This study also found that the biochar treatments reduced the ammonium nitrogen content in the soil at the harvest period, and the alkali-hydrolyzable nitrogen content of the soil at the harvest period decreased significantly with the decrease in nitrogen application rate. This may be attributed to more nitrogen being absorbed and utilized by the plants, which is of significance in reducing nitrogen loss and alleviating soil compaction and acidification[37]. Further regression analysis of soil nitrogen content and tobacco nitrogen metabolism indicators revealed a linear increase between the whole plant nitrogen accumulation and nitrogen uptake efficiency and the nitrate nitrogen content, while the ammonium nitrogen content showed a negative growth trend with nitrogen uptake efficiency. This is consistent with previous research, suggesting that tobacco prefers nitrate nitrogen in nitrogen source absorption[38]. This indicates that applying biochar can significantly increase the nitrate nitrogen content in the soil, thereby ensuring the accumulation of nitrogen in tobacco plants[16].
Previous research by our team has revealed that biochar amendment in the tobacco-rice rotation system can significantly reduce soil N2O emissions in two seasons[39]. Chen et al.[40] found that the application of biochar significantly increased soil AOA abundance. Prommer et al.[41] found that the application of biochar significantly enhanced soil nitrification. Here, biochar treatments significantly increased the abundance of the ammonia oxidation gene AOA-amoA compared to the control treatment while reducing the abundance of the denitrification gene norB. norB is responsible for the intensity of N2O conversion in nitrogen cycling. Therefore, this study further validates the research results of our team. Due to the potential correlation between the abundance of nitrogen cycling functional genes and nitrogen balance, high input of nitrogen fertilizer may lead to an increase in the abundance of nitrification and denitrification-related functional genes, resulting in potential risks such as nitrogen loss and decreased nitrogen fertilizer uptake efficiency[42]. In this experiment, as the nitrogen application rate decreased, the abundance of nitrogen cycling functional genes in the nitrogen reduction treatments showed varying degrees of decline, which is beneficial in reducing the risk of nitrogen loss in the soil. However, the nitrogen reduction treatments of 10%~20% still showed a significant increase in the abundance of nitrification gene nxrA, nitrogen fixation gene nifH, ammoniation gene UreC, and organic nitrogen synthesis gene gdh compared to the control treatment (T0). This may be attributed to the application of biochar, which provides a favorable habitat for microorganisms, enabling the nitrogen cycling functional genes to maintain a higher abundance even under a reduced nitrogen supply[43]. The specific mechanisms behind this phenomenon require further investigation incorporating microbiological studies. Random forest model analysis revealed that nitrogen cycling functional genes accounted for a total of 90.08% of the variability in tobacco nitrogen uptake efficiency, indicating that these genes can effectively predict the level of nitrogen absorption and utilization in tobacco. Among them, nitrogen fixation gene nifH, organic nitrogen synthesis gene gdh, and ammoniation gene UreC showed a highly significant correlation with nitrogen uptake efficiency, while nitrification gene nxrA showed a significant correlation. The transformation of different forms of nitrogen in the soil relies on the mediation of nitrogen cycling functional genes[44]. Liu et al.[45] demonstrated through redundancy analysis that soil nitrate nitrogen and ammonium nitrogen content were most closely associated with the abundance of nitrogen cycling functional genes in the soil. Mantel test results showed the atalkali-hydrolyzable nitrogen and nitrate nitrogen content exhibited a highly significant positive correlation with the abundance of ammoniation gene UreC, nitrification gene nxrA, and nitrogen fixation gene nifH. This may explain why the nitrogen uptake efficiency maintained a higher level even under the nitrogen reduction treatments.
Changes in soil carbon content can have a significant impact on nitrogen cycling[46]. Previous studies have shown that exogenous carbon input can enhance plant growth and ecosystem carbon sequestration. It can also improve nitrogen availability by increasing biological nitrogen fixation and mineralization, while reducing nitrogen leaching. In addition, exogenous carbon input can increase soil microbial abundance, significantly increasing the abundance of bacteria, methanogens, methane-oxidizing bacteria, and ammonia-oxidizing bacteria in the 10−20 cm soil layer[47, 48]. When biochar is applied to the soil as an exogenous material, the organic carbon it carries can serve as a carbon source for nitrogen cycling microorganisms, promoting their growth and reproduction. Our findings showed that the application of biochar can significantly increase soil pH and organic matter content. Further regression analysis reveals that organic matter content significantly influences the abundance of gdh, nxrA, and nifH genes, making it one of the most important physicochemical properties affecting the abundance of nitrogen cycling functional genes. Additionally, pH also significantly influences the abundance of ammonia oxidation genes AOA-amoA and denitrification gene norB. In this experiment, the improvement of soil bulk density and porosity significantly increased the abundance of nitrification gene nxrA, possibly due to the provision of ample colonization space and oxygen to nitrifying microorganisms by biochar[41]. These results indicate that the application of biochar can enhance the abundance of nitrification genes, reduce the abundance of denitrification genes, and consequently increase the content of alkaline nitrogen and nitrate nitrogen in the soil, ultimately improving the nitrogen absorption and utilization efficiency of tobacco plants. Although moderate nitrogen reduction reduces the abundance of nitrogen cycling functional genes to some extent, compared to the control treatment, the abundance of nitrification gene nxrA, nitrogen fixation gene nifH, and ammonification gene UreC still showed significant improvement.
-
The findings of the two-year experiment showed that reduced nitrogen fertilizer application combined with biochar had a significant impact on nitrogen accumulation in tobacco plants. The trend of nitrogen accumulation was observed as T1 > T2 > T3 > T4, with T0 being the lowest. The use of biochar in combination with a 10%−20% nitrogen reduction (T2 and T3) does not show a significant difference in nitrogen uptake efficiency compared to the conventional nitrogen application treatment (T1). In the second year of the experiment, the nitrogen harvest index in T2 and T3 increased by 3.85% and 5.78%, respectively, compared to T1. The possible reason for maintaining a higher nitrogen uptake efficiency under nitrogen reduction conditions is the application of biochar, which improves soil pH, organic matter content, and soil porosity, thereby enhancing the abundance of nitrogen cycling genes in the rhizosphere soil and increasing the content of alkaline nitrogen and nitrate nitrogen in the soil. This experiment suggests that under the condition of full-surface application of biochar (30 t·ha−1), a reduction of 10%−20% in nitrogen fertilizer can be achieved compared to conventional nitrogen application, thus sustainably promoting the production of tobacco.
-
The authors confirm contribution to the paper as follows: Conceptualization, Methodology, Investigation, Writing − original draft, Writing − review & editing: Zhang B; investigation, formal analysis, visualization: Tang L, Wang Y, Yang M, Pan R, Pan M, Chen X, You L; Investigation, Data curation: Huang J, Lin W. All authors reviewed the results and approved the final version of the manuscript.
-
The data in this paper are free from any conflict of interest. The data that support the findings of this study are available from the corresponding author upon reasonable request.
This research was supported by the Fujian Branch of China National Tobacco Corporation, China (2019350000240143).
-
The authors declare that they have no conflict of interest. Wenxiong Lin is the Editorial Board member of Technology in Agronomy who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Supplemental Table S1 qRT-PCR primers for key functional genes of nitrogen cycle in rhizosphere soil.(2022).
- Supplemental Table.S2 Significance analysis of the difference in abundance of functional genes of nitrogen cycle between different treatments and control (%) (2022).
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang B, Tang L, Wang Y, Yang M, Pan R, et al. 2023. Effect of reduced nitrogen fertilizer application combined with biochar on nitrogen utilization of flue-cured tobacco and its association with functional gene expressions of the nitrogen cycle in rhizosphere soil. Technology in Agronomy 3:12 doi: 10.48130/TIA-2023-0012
Effect of reduced nitrogen fertilizer application combined with biochar on nitrogen utilization of flue-cured tobacco and its association with functional gene expressions of the nitrogen cycle in rhizosphere soil
- Received: 21 July 2023
- Accepted: 05 October 2023
- Published online: 31 October 2023
Abstract: Studies have shed light on the impact of the co-application of inorganic fertilizer and biochar on soil fertility, health, and crop growth performance and yield. However, insufficient literature exists regarding the appropriate nitrogen reduction ratio for enhancing soil quality and maximizing crop nitrogen utilization following the application of biochar in a continuous tobacco-rice rotation field. Here, we explored nitrogen absorption and utilization patterns of tobacco crops, as well as the response characteristics of functional genes related to soil nitrogen cycling subjected to the interaction of reduced nitrogen utilization ratios following biochar application in a long-term tobacco-rice rotation field. The results showed that the treatments with 10% (T2) and 20% (T3) nitrogen reduction combined with biochar (30 t∙ha−1) promoted nitrogen utilization efficiency and nitrogen harvest index of tobacco plants. In the second year of the experiment, T2 and T3 significantly increased the nitrogen harvest index by 3.85% and 5.78% compared with the conventional nitrogen application treatment (T1), respectively. We believe that the increase in abundance of nitrification, nitrogen fixation, and ammonification genes, including nxrA, nifH, and UreC in the rhizosphere soil, precipitate the high nitrogen absorption and utilization efficiency in the biochar combined with nitrogen reduction treatments, respectively. This suggests that biochar application at a rate of 30 t·ha−1, nitrogen fertilizer usage can be reduced by 10% and 20% to achieve optimal and sustainable tobacco production.
-
Key words:
- Flue-cured tobacco /
- Biochar /
- Nitrogen reduction /
- Nitrogen metabolism /
- Nitrogen cycle function gene













