-
Dendrobium belongs to the Orchidaceae family, one of the most evolutionarily advanced and species-rich families among angiosperms. Plants in the genus Dendrobium not only possess medicinal value but also exhibit unique floral shapes and vibrant colors, making them highly ornamental[1]. There are three propagation methods for plants in Dendrobium: seed germination, cane or stem cutting, and tissue culture[2]. Seed germination is usually used for breeding as the germination rate of orchid seeds is extremely low in both natural habitats and laboratory environments, and often require fungal symbionts to facilitate germination. Research indicates that the presence of fungal mycorrhizas can significantly enhance the germination rate of Dendrobium[3]. The duration of vegetative propagation methods, such as clonal cutting, varies considerably among species, typically requiring between one and six months. This lengthy duration and low efficiency represent a significant drawback of this asexual propagation technique[2]. In contrast, tissue culture propagation allows for various choices of explants and is not restricted by seasonal and environmental conditions, enabling high-efficiency production year-round[4]. For instance, a propagation system for Dendrobium wilsonii Rolfe was established using germinated seeds as explants, and multiple shoots were induced after culture[5]. Mentioning to the other influencing factors, melatonin was reported to promote the proliferation of protocorm-like bodies (PLBs) in Dendrobium 'Sabin Blue'[6], and DoERF5 regulated the regeneration of pseudobulbs by activating DoSTM expression to facilitate the clonal propagation of Dendrobium officinale[7].
Somatic embryogenesis under in vitro conditions exploits cell totipotency, resulting in low chimera frequency, high regenerant numbers, and minimal somaclonal variation. Thus, it represents a superior plant regeneration system compared to organogenesis in orchids[8]. A comparative micropropagation study on several Dendrobium cultivars, focusing on PLB production and somatic embryo induction, demonstrated the superiority of somatic embryo induction, yielding over one million plantlets, while the 'mother' PLBs gradually senesced and browned[9]. Various explants, including leaf and seed-derived protocorm, have been induced to undergo somatic embryogenesis on media supplemented with TDZ[10]. Phytosulfokine (PSK), a plant peptide hormone functioning in plant growth, development, and stress response[11,12], also plays an important role in embryogenic callus induction. The addition of PSK to the culture medium of Cunninghamia lanceolata (Chinese fir) enhances the induction of embryogenic callus, providing theoretical and technical support for establishing efficient somatic embryogenesis systems[13]. Additionally, in Dendrobium primulinum, a regeneration system using stem segments and shoot tips was successfully established by optimizing MS basal medium supplemented with natural organic additives (40.0 g/L banana homogenate and 30.0 g/L potato homogenate) and a combination of plant growth regulators (1.0 mg/L 6-BA, 0.5 mg/L KT, and 2.0 mg/L NAA)[14], indicating that the supplement of nutrient-rich natural additives can improve the efficiency of embryogenic callus induction, providing healthier material for seedling production in large-scale Dendrobium propagation.
The Dendrobium kingianum, native to northern Australia, is one of the few aromatic species within its genus and holds considerable economic value. However, the challenges in seedling propagation and the lengthy cultivation cycle have resulted in high prices of this potted flower, limiting the market potential for Dendrobium kingianum[15]. Previous studies stated that various parts of the Dendrobium plants, including shoot tips, leaves, flower stalks, nodes and internodes, pseudobulb segments, and rhizome buds, can serve as explants for micropropagation[2]. Therefore, the leaves and roots of sterilized-tissue-cultured plants were first used to induce embryogenic callus on CIM (callus induction medium) containing 0.2 mg/L NAA, 1.5 mg/L 6-BA, and 10−6−10−8 mol/L PSK. The explants were then cultured in the dark at 23 ± 2 °C for callus induction. The petri dishes were then transferred to light conditions (40 μmol·m−2·s−1, 16 h light/8 h dark, 23 ± 2 °C) when callus was visible. A few calluses wereinduced after a long culture period, and the tissues browned and died (Supplementary Fig. S1). To obtain more regenerative tissue for further experiments, pseudobulbs were cultured on MS medium supplemented with NAA and 6-BA at different concentrations to induce lateral buds. Medium consisting of 1/2MS, 20 g/L sucrose, 3 g/L Phytagel, 1.5 mg/L NAA, 5 mg/L 6-BA, 2 mg/L KT, 1.5 mg/L peptone, and 200 mL/L coconut water can induce the induction rate and promote the growth of rhizome bud (Fig. 1a). Twenty-day-old lateral buds were cut into slices with a sharp, sterilized blade and then cultured on CIM. Round, condensed, and yellow embryogenic calluses were observed after about 60 d of induction, and they proliferated faster with 10−7 mol/L PSK supplementation. The embryogenic callus further proliferated, and around 90 d after induction, large masses of yellow embryogenic calluses were obtained (Fig. 1b, c). Through paraffin sectioning of the embryogenic callus, the presence of typical somatic embryo structures was confirmed, such as the cotyledonary embryo stage, demonstrating that regeneration occurred via the somatic embryogenesis pathway (Fig. 2a, b).
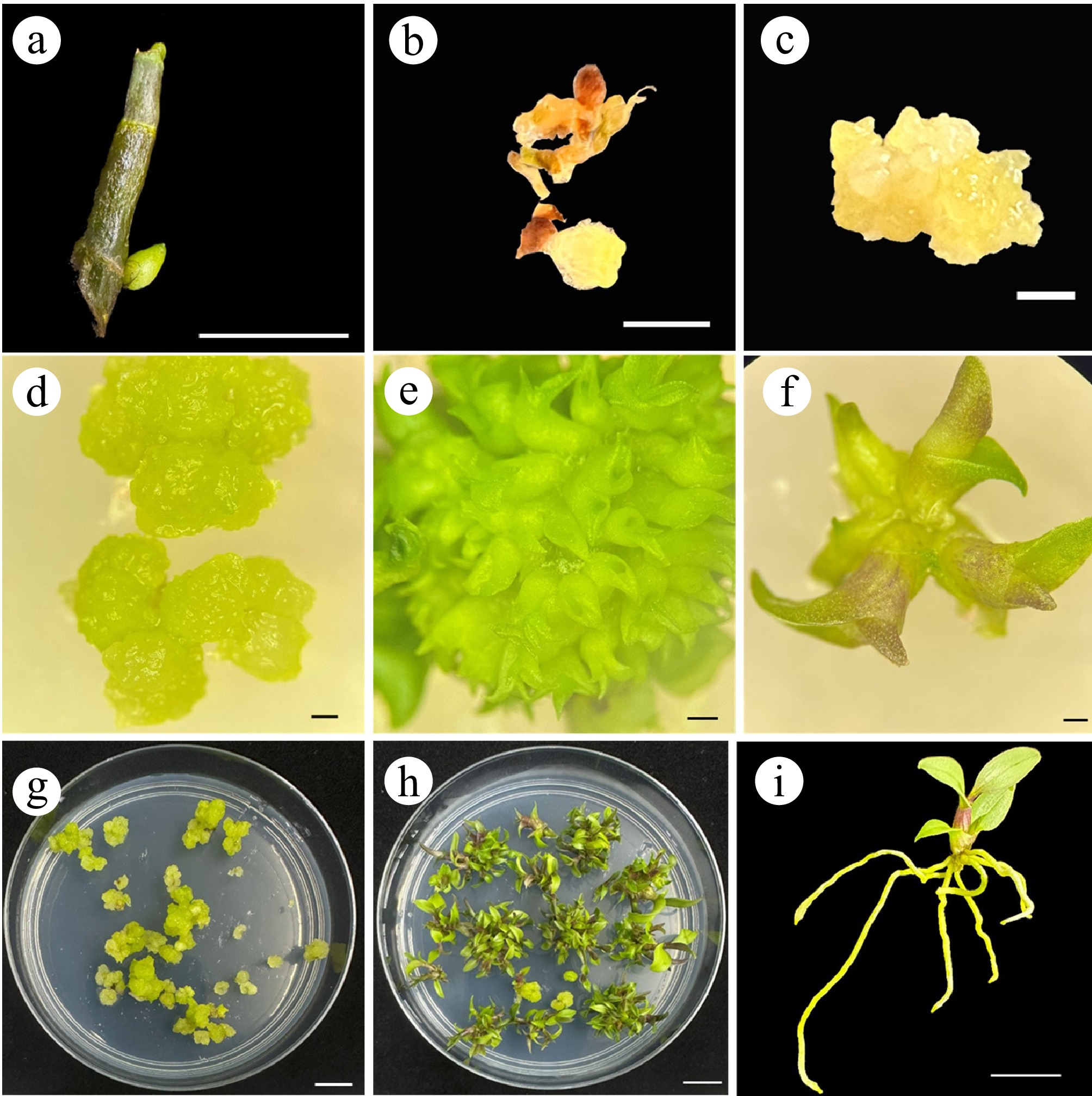
Figure 1.
Main steps of the somatic embryogenesis for Dendrobium kingianum propagation.
(a) The induction of the lateral bud. (b), (c) The induction of embryogenic callus. (d)–(f) Differentiation of somatic embryos. (g), (h) The proliferation of embryogenic callus and propagation of seedlings. (i) Plantlet with robust roots. Scale bar = 1 cm.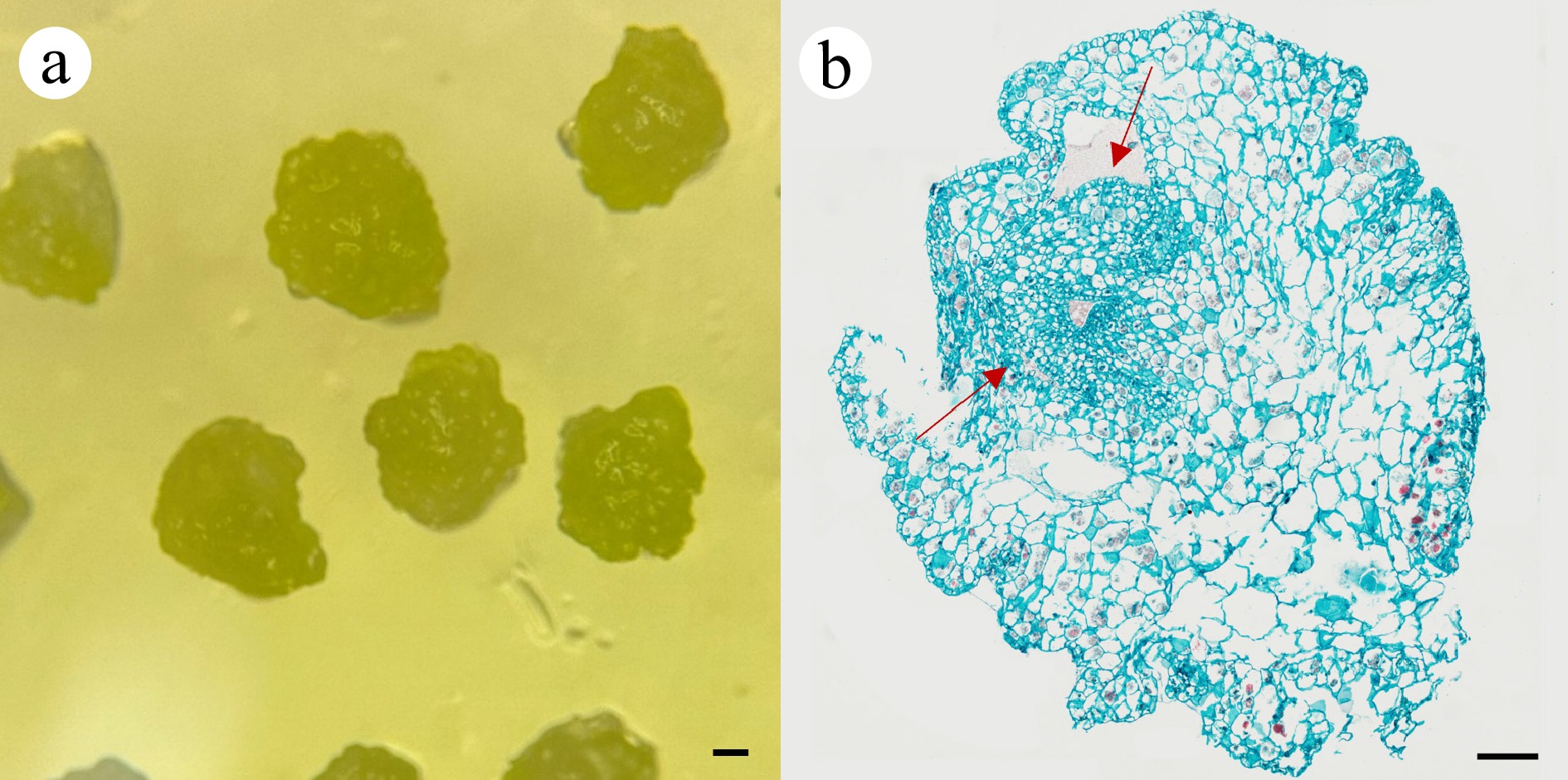
Figure 2.
Histological observation of embryogenic callus.
(a) Embryogenic callus collected for paraffin section. (b) A typical somatic embryo structure, as seen by paraffin section, stained with Safranin and Fast Green. Red arrows indicate a somatic embryo with condensed cells. Scale bar = 200 µm.To establish an efficient somatic embryogenesis system, various media were tested for somatic embryo development, which includes different media bases (adding 4.74 g/L or 2.37 g/L MS powder, replacing MS powder with 1 g/L Hyponex No.1 and 1 g/L Hyponex No.2), as well as different concentrations of phytohormones, and the addition of other nutrients. The differentiation of embryogenic callus was generally limited on medium G1 (MS + 0.1 mg/L NAA + 1.5 mg/L 6-BA + 30 g/L sucrose + 7 g/L agar), resulting in the formation of a few pale-yellow seedlings on only one callus (Supplementary Fig. S1d). Medium G2 (1/2 MS + 0.1 mg/L NAA + 1.0 mg/L 6-BA + 30 g/L sucrose + 7 g/L agar) showed a slightly better somatic embryo induction, with embryos growing faster. However, only one callus underwent the differentiation process, and the rest of the callus died totally (Supplementary Fig. S1e). Malformed organs were observed on medium G3 (1/2 MS + 30 g/L sucrose + 7 g/L agar), indicating the essential function of phytohormones in somatic embryogenesis (Supplementary Fig. S1f). The best differentiation results were observed on medium G4 (1.0 g/L Hyponex No.1 + 1.0 g/L Hyponex No.2 + 15 g/L sucrose + 3 g/L Phytagel + 2 mg/L 6-BA + 0.5 mg/L IBA + 10 g/L corn starch + 200 mL/L coconut water), as more somatic embryos were formed on this medium. The differentiated SEs were healthy, uniform, and displayed robust growth (Fig. 1d, e). The developed somatic embryos, upon reaching a certain developmental stage, will automatically detach from the callus and establish a robust and healthy root system, thereby completing the development process from early SE to a robust seedling (Fig. 1f, i). The mature seedlings can be hardened off and transplanted into the soil 30 d after rooting, facilitating an efficient system for the rapid in vitro propagation of Dendrobium (Fig. 1g, h). The embryogenic callus exhibited sustained proliferation capacity on MS medium, and multiple plantlets were successfully regenerated from the original somatic callus through several subculture cycles, indicating the successful establishment of a seedling propagation system for Dendrobium kingianum (Supplementary Fig. S2).
Various tissues of Dendrobium can serve as explants for micropropagation through direct or indirect induction. During explant selection, it was observed that conventional tissues such as leaves and roots exhibited poor induction responses, characterized by severe browning and low induction rates, indicating a limited capacity for cellular dedifferentiation. In contrast, pseudobulb tissues demonstrated a markedly higher regenerative potential, readily forming compact, pale-yellow embryogenic callus. This superior response may be attributed to the presence of undifferentiated or meristematic cell populations and favorable endogenous hormone levels, which facilitate the reprogramming of cell fate and confer a higher capacity for somatic embryogenesis. These findings are consistent with previous reports in various orchid species, where pseudobulbs have been identified as preferred explants for in vitro regeneration[16,17]. For instance, apical bud materials were cultured for shoot regeneration in D. primulinum[14], and 4.75% of the explants were induced into embryogenic callus. Likewise, only one explant was induced into embryogenic callus in our case, indicating the difficulties in embryogenic callus induction. According to the D. primulinum case and our work, natural nutrient substances including banana, potato, and coconut water, seem to be favorable supplements in the induction medium.
In addition, chemicals like PSK also promote somatic embryogenesis and organogenesis by modulating key regulatory genes and enhancing cellular reprogramming[18,19]. The results showed that the addition of 10−7 mol/L PSK to the basal medium facilitated the induction and accumulation of embryogenic callus. PSK has been reported to participate in the cellular processes of various plant species, including cell division, dedifferentiation, and redifferentiation. Its promotive effects on regeneration are likely mediated through enhancement of embryogenic competence and activation of key cell cycle regulators[18−20]. The demonstrated effectiveness of PSK in this study suggests that it may serve as a crucial exogenous signaling molecule in somatic embryogenesis, with potential for further application in future optimization efforts.
HTML
This work was supported by Yunnan Xingdian Talents—Youth Special Project (Grant No. XDYC-QNRC-2022-0731), Yunnan Seed Laboratory Project (Grant No. 202205AR070001-2024KF01), and Yunnan Xingdian Talents—Special Selection Project for High-level Scientific and Technological Talents and Innovation Teams-Team Specific Project, Xingdian Talent Support Project (Grant No. CYRC2020004).
-
The authors confirm their contributions to the paper as follows: experiments performed: Liu M, Jin C (lead); writing - draft manuscript preparation: Liu M, Jin C; data analysis and visualization: Yuan L; experimental materials preparation: Wu L, Lu Z;experiments supervision: Yang C, Jin C; experiments guidance: Yang. All authors reviewed the results and approved the final version of the manuscript.
-
The data supporting the findings of this study are available within the article and its supplementary information files.
-
The authors declare that they have no conflict of interest.
- Supplementary Fig. S1 Embryogenic callus induction with different explants and somatic embryogenesis on different mediums. (a−c) embryogenic callus induction from leaf (a), root (b) and lateral bud (c); (d−f) somatic embryogenesis on G1 (d), G2 (e) and G3 (f) medium at about 45 d. Scale bar = 1 cm.
- Supplementary Fig. S2 Scale production of Dendrobium kingianum seedlings in laboratory. (a) proliferation of embryogenic callus; (b−c) somatic embryo differentiation; (d−e) seedling culture; (f) mature plant growth. Scale bar = 1 cm.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Yunnan Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Liu M, Yuan LL, Wu L, Lu Z, Yang C, et al. 2025. The establishment of a micropropagation system for Dendrobium kingianum via somatic embryogenesis. Agrobiodiversity 2(3): 56−58 doi: 10.48130/abd-0025-0007 |













