-
Neddylation is a post-translational modification process that involves the covalent conjugation of NEDD8 to substrate proteins, most notably cullin proteins. This enzymatic cascade is mediated by three classes of enzymes: NEDD8-activating enzyme (E1), NEDD8-conjugating enzyme (E2), and NEDD8 ligating enzyme (E3), which is implicated in a spectrum of pathological conditions, including metabolic dysfunction-associated steatotic liver disease (MAFLD), diabetes, cardiovascular disease, and neurodegenerative disorders[1,2]. Given that the neddylation is essential for Cullin RING E3 ligases (CRLs) activity, it precisely regulates the ubiquitination and degradation of substrate proteins to maintain intracellular homeostasis, presenting neddylation as a highly promising frontier for drug discovery. The pharmacotherapeutic potential of neddylation inhibition has been demonstrated by E1 inhibitors such as MLN4924 and TAS4464, which are going through clinical trials for cancer treatment (Fig. 1a)[3]. Additionally, the progression of metabolic diseases, such as MAFLD and obesity, are accompanied by elevated neddylation activity in liver and adipose tissue, while neddylation E1 inhibitors indeed significantly ameliorate obesity and MAFLD[4−7]. However, pan-neddylation inhibitors non-selectively inhibit all cullin proteins, which inevitably causes unexpected side effects[8]. Identification of the individual cullin functions in metabolic organs or specific cells and discovery of the targeted agents have achieved great significance for metabolic disease drug discovery.
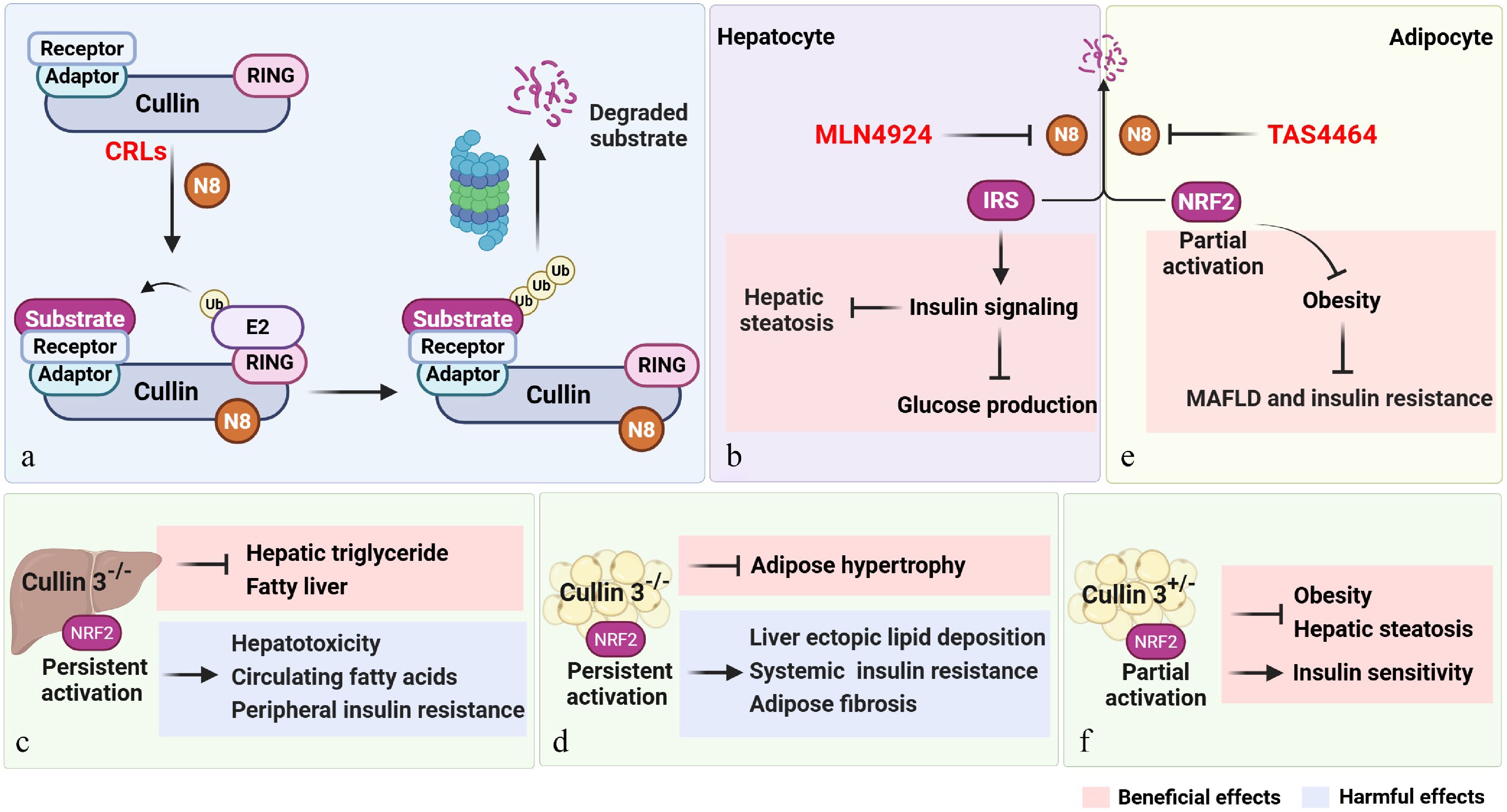
Figure 1.
Cullin 3 and neddylation inhibitors exert dual metabolic effects in hepatocytes and adipocytes. (a) Structure of CRLs and neddylation processes involved in substrate recognition and degradation. (b) MLN4924 inhibits neddylation process, thereby increasing the stability of IRS to alleviate hepatic steatosis. (c) and (d) Hepatocyte or adipocyte-specific cullin 3 knockout induces persistent NRF2 activation, resulting in both beneficial and detrimental metabolic phenotypes. (e) Neddylation inhibitor TAS4464 stabilize and partial activate NRF2, promoting lipolysis and ameliorating obesity. (f) Adipose-specific cullin 3 heterozygous knockout induces intermittent NRF2 activation, leading to improved insulin sensitivity, obesity as well as hepatic steatosis. The graphic was created with BioRender.com (agreement number: KV29492SLA).
-
A recent study from Prof. Tiangang Li's laboratory at the University of Oklahoma Health Sciences Center demonstrates that partial knockout of cullin 3 in adipose tissue alleviated diet-induced obesity and is responsible for the anti-obesity effects of pan-neddylation inhibitors[9]. These findings extend the earlier work of two laboratories: (1) by stabilizing hepatic insulin receptor substrate (IRS) and reducing blood glucose, hepatocyte cullin 3 knockdown recapitulates the therapeutic effect of MLN4924; (2) liver-specific cullin 3 knockout results in the rapid resolution of fatty liver in obese mice[10,11]. Together, these findings systematically elucidate the cell-type-specific functions of cullin 3 in metabolic disease and provide novel insights for developing neddylation-targeting drugs.
-
Ubiquitination-mediated degradation of IRS is the driving factor for insulin resistance. Although several E3 ubiquitin ligases have been demonstrated to promote IRS degradation, it remains unclear which cullin is involved in this process. Previous work from Prof. Tiangang Li's laboratory has uncovered that hepatocyte cullin 1 and cullin 3 have interacted with IRS, which is responsible for stabilizing IRS in vitro. However, knockdown of liver cullin 3 has increased IRS levels and improved glucose tolerance, recapitulating the insulin-sensitizing effect of MLN4924 in vivo (Fig. 1b)[11]. These findings have reinforced the concept that pharmacological neddylation inhibition is a promising therapeutic strategy for fatty liver disease as well as diabetes management[4−6,12]. Given that insulin resistance is the hallmark feature in the development of metabolic fatty liver disease, it is rational to investigate the involvement of hepatocyte cullin 3 in hepatic lipid metabolism with tissue-specific knockout mice.
-
As expected, hepatocyte cullin 3 knockout mice alleviated hepatic triglyceride accumulation and stabilized IRS levels, demonstrating a beneficial effect of hepatocyte-specific cullin 3 deletion. Contrary to expectations, the knockout also induced hepatic lipotoxicity, elevated circulating fatty acids, and peripheral insulin resistance, revealing a detrimental phenotype[10]. While NRF2 activation is typically viewed as a protective mechanism and a therapeutic target for steatosis and hyperglycemia, its chronic activation in this context is paradoxically associated with liver injury, exacerbated steatosis, and systemic metabolic dysregulation[13]. Subsequent mechanistic investigations demonstrated sustained NRF2 activation resulting from cullin 3 knockout, which is the primary cause of the detrimental effects of cullin 3 loss. Therefore, partial inhibition of cullin 3 is sufficient to restore NRF2 physiological levels that alleviate hepatic steatosis and improve systemic metabolism without triggering deleterious outcomes, while cullin 3 inactivation drives NRF2 overactivation, causing metabolic maladaptation. The contrasting outcomes between neddylation inhibitors and hepatocyte-specific cullin 3 knockout highlight the need for careful modulation of neddylation inhibition to avoid adverse compensatory effects (Fig. 1c).
-
While the anti-obesity properties of pan-neddylation inhibitors have been previously reported, specific effects on adipose tissue biology and functional contributions of individual cullin proteins remain poorly defined[5]. In the latest article, Prof. Tiangang Li's group further identified distinct roles for specific cullins in adipogenesis: cullin 1 and cullin 3 function as inhibitors of this process, while cullin 4A exerts a promotive effect in vitro. In the meantime, mice fed Western diet showed significance body weight gain reduction following adipocytes cullin 3 knockouts using multiple techniques, manifesting the key role of adipocyte cullin 3 in fat accumulation and obesity progression. Nevertheless, comprehensive deletion of cullin 3 might be linked to unfavorable reactions, ranging from hepatic lipid accumulations, insulin resistance and other metabolic syndrome-related criteria. These effects could be due to the dysfunctional capacity of excess lipid buffering control of the adipose tissue, triggering lipid reallocation toward metabolic-sensitive organs, such as the liver. Furthermore, the above reported alterations were found to be accompanied by inflammatory mediators and immune chemokines overexpression, as well as collagen deposition within adipose tissue, that directly resembles prolonged activation of NRF2 signaling pathway (Fig. 1d). In contrast, pharmacological approaches to inhibit neddylation using TAS4464 generated dual achievements, by influencing cullin 3 deactivation, as well as induction of partial and transient suppression. Therefore, the overall outcomes ranging from attenuated obesity, hepatic steatosis mitigation, and improved insulin sensitivity (Fig. 1e). This divergence in outcome indicates that the degree and duration of cullin 3 inhibition critically influence adipose tissue responses.
Consistent with this perception, rodents carrying a heterozygous deletion of cullin 3 exhibit a more favorable metabolic phenotype, depicted by reduced adiposity, diminished hepatic lipid accumulation, and improved insulin sensitivity (Fig. 1f). Although heterozygous deletion might lead to an approximately 50% cullin 3 deactivation, it is still adequate to decelerate adipose expansion without abolishing lipid storage capacity[9]. This moderated reduction supports adaptive adipose remodeling and mirror the therapeutic action of TAS4464. Taken together, the findings confirm adipocyte cullin 3 as a viable therapeutic target, and indicate that partial inhibition, rather than complete knockout modulation, might be a crucial strategic key to harmonizing efficacy and safety in cullin 3-based treatments.
-
Collectively, these findings highlight the context-dependent functions of cullin 3 in liver and adipose tissue and illustrate the translational challenges associated with manipulating cullin neddylation. In hepatocytes, cullin 3 contributes to insulin resistance through regulation of IRS stability, whereas in adipose tissue it influences adipogenic capacity and lipid handling. Complete loss of cullin 3 improves select metabolic parameters but concurrently disrupts systemic homeostasis, in part through sustained activation of NRF2 signaling. Across tissues, persistent NRF2 activation emerges as a maladaptive response that interferes with lipid synthesis and storage. These observations indicate that therapeutic benefit is more likely to arise from partial and controlled attenuation of cullin 3 activity, rather than from its complete inhibition, thereby permitting metabolic improvement while preserving essential regulatory functions.
The above evidences so far place particular emphasis on the involvement of cullin 3 in metabolic regulation, alongside in vitro experimental observations indicate that interfering with cullin 1 produces distinct metabolic consequences. Namely, hepatocytes cullin 1 depletion slows the degradation of IRS, whereas it is loss in adipocytes hinders adipogenic differentiation. However, these findings are not fully recapitulated in vivo, as partial deletion of cullin 1 in hepatocytes does not lead to a substantial rise in IRS levels or a clear enhancement of glucose tolerance. One potential explanation of this divergence between in vitro and in vivo outcomes probably attributed to the functional redundancy within Cullin RING E3 ligase (CRL) complexes, in which cullin 1 reduction could be compensated by other E3 ligases, thus IRS turnover preserved. Consequently, the slight increase in IRS that follows cullin 1 loss seems insufficient to trigger a strong hypoglycemic effect. Moreover, a profound gap in the current research is the lack of an adipocyte-specific cullin 1 knockout model, as the entire cullin 1 contribution in the adipose tissue functions and metabolic regulations could not be fully addressed. Thus, establishment of such models is of utmost necessary in determination of cullin 1 tissue-specific effects, resemble to those seen with cullin 3.
CRLs considered as multi-protein apparatuses, being built from a cullin scaffold, a RING-box protein, various adaptor components, and receptors that identify substrates. Recent innovation in high-throughput screening, structure-based design, and AI-driven drug discovery has augmented the search potential candidates that selectively inhibit CRLs. Despite these technology advancements, direct pharmacological targeting of the neddylation pathway remains challenging, which might be linked to the lacking of suitable binding sites, as well as CRL structural complexity. In the search of promising alternatives, Defective in Cullin Neddylation 1 (DCN1), a co-E3 ligase, has emerged as an alternative target that promotes CRL activation via bridging the neddylation E2 enzyme UBE2M with cullin proteins[14]. Notably, disruption of DCN1–UBE2M interaction by small molecules that act as DCN1 antagonist, initiate selective attenuation of the cullin 1 and cullin 3 neddylation, and their therapeutic potential demonstrated in models of chronic disease[14,15]. Given the essential role of cullin 1 and cullin 3 in both hepatocytes and adipocytes, it remains important to investigate whether targeting DCN1 could be a viable strategy for metabolic diseases. Otherwise, to avoid CRL dysfunction caused by extensive inhibition of cullin 3, it is of great significance to identify specific CRL complexes to achieve precise intervention.
In summary, these existing evidences underscore the paradoxically effects of cullin 3 in liver and adipose and highlight the challenge of targeting neddylation for pharmacotherapy. Through interacting and interfering with IRS stability, cullin 3 deteriorates hepatocyte insulin resistance and promotes adipogenesis in adipose tissue. Although hepatocyte or adipocyte cullin 3 knockout improves some metabolic indicators, its main consequence is to disrupt the overall metabolic homeostasis. This phenomenon may be related to the sustained NRF2 activation, in which overactivation of NRF2 acts as a maladaptive response that interferes with lipid synthesis and storage. Hence, partial and intermittent activation of cullin 3 could achieve higher therapeutic benefits, thereby enabling metabolic improvement while preserving essential regulatory functions.
-
Not applicable.
-
The authors confirm contributions to the paper as follows: study conception, manuscript revision and supervision: Yu B, Qin T; draft manuscript preparation, figure creation: Yuan Z, Wang Z; critical revision and expert input: Yuan Z, Wang Z; funding acquisition Yu B, Yuan Z. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
-
This study was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (Grant No. 2023ZD0507700), National Natural Science Foundation of China (Grant No. 82304587), Natural Science Foundation of Henan Province (Grant Nos 242301420005 and 252300421243), Young Elite Scientists Sponsorship Program by Henan Association for Science and Technology (Grant No. 2025HYTP076), Key Research Project for Basic Research in Henan Province Universities (Grant No. 25ZX001), Scientific Research Innovation Capability Support Project for Young Faculty (Grant No. SRICSPYF-BS2025078), China Postdoctoral Science Foundation (Grant No. 2024M750816), State Key Laboratory of Metabolic Dysregulation and Prevention and Treatment of Esophageal Cancer (Grant No. 2025SGAQZ-MS-01) and Henan Province Clinical Medical Scientists Training Program (Grant No. HNCMS202410).
-
The authors declare no conflict of interest.
-
#Authors contributed equally: Ziqiao Yuan, Ziwei Wang
- Copyright: © 2026 by the author(s). Published by Maximum Academic Press on behalf of China Pharmaceutical University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yuan Z, Wang Z, Qin T, Yu B. 2026. Cell-type-specific role of cullin 3 in adipocytes and hepatocytes: metabolic implications of neddylation inhibition. Targetome 2(1): e002 doi: 10.48130/targetome-0025-0013
Cell-type-specific role of cullin 3 in adipocytes and hepatocytes: metabolic implications of neddylation inhibition
- Received: 06 November 2025
- Revised: 15 December 2025
- Accepted: 26 December 2025
- Published online: 29 January 2026
-
Key words:
- Neddylation /
- Cullin 3 /
- Obesity /
- Fatty liver disease /
- NRF2