-
Tillering onion (A. cepa) is a herbaceous species within the Allium genus, belonging to the Liliaceae family, and represents a specific variety of onion. These onions exhibit cold resistance and are extensively cultivated in Heilongjiang and Jilin Provinces (China)[1]. Currently, the majority of germplasm resources utilized in the cultivation of tillering onions are derived from farm varieties, predominantly employing bulbs as the reproductive structures. A significant challenge in production arises from the difficulty of sexual reproduction in tillering onions, which hampers the plants' ability to bolt and flower. Prolonged reliance on asexual reproduction has led to substantial virus accumulation and infection, resulting in stunted growth, reduced plant height, fewer tillers, and a marked decline in both yield and quality[2]. The application of tissue culture technology to produce test tube bulbs can enhance bulb quality, thereby improving the overall quality of tillering onions[3]. Concurrently, the optimization of the bulb disk tissue culture system for tillering onions, along with the refinement of propagation and storage conditions, offers theoretical support for industrial-scale seedling breeding.
As a significant vegetable crop in the Northeast, tillering onions are crucial to the livelihoods of many. The bulbs of tiller onion contain volatile sulfide with a special spicy taste, including flavonoids, phenolic compounds, and a variety of nitrogen compounds, which are suitable for fresh food and processing and have high utilization value[1]. It is one of the emerging characteristic vegetables in China. Onions are widely planted in Heilongjiang, Jilin, and other places. As a regional characteristic vegetable, they are called 'three spicy' together with pepper and garlic and are widely exported to Southeast Asia, Russia, Japan, and South Korea[4]. This research aims to establish a more comprehensive and dependable methodology for the cultivation and propagation of tillering onions by optimizing the secondary tissue culture and preservation protocols. Currently, studies conducted by previous researchers have developed a rapid propagation tissue culture system for the Liliaceae family and an effective regeneration system for lilies[5−7]. Additionally, Xu & Chen, and Su et al., have created a tissue culture and rapid propagation framework specifically for tillering onions[8,9]. Building on this foundation, the present study undertakes a thorough optimization analysis of growth height and longitudinal cutting ratios.
Restricting growth from the body is a way to ensure the maintenance, and regeneration potential of cultivation and regeneration, and significantly reduce the method of preservation time, and reduce maintenance costs by significantly slowing the physiological metabolism process of the plant[10,11]. Growth inhibitors are two common preservation methods. Among them, mannitol is one of the common growth delay agents and inhibitors. Research results from other researchers all showed that in the various chemical reagents selected, the preservation effect of the treatment with mannitol is mainly manifested in its minimum mutation, and morphological mutations can be restored in the secondary generation[12−14]. Current investigations into mannitol preservation media within horticulture predominantly concentrate on floral species, including phalaenopsis and chrysanthemums[15,16], as well as certain fruit-bearing trees like citrus and cherries[17,18]. The vegetable sector has also been explored, particularly regarding the optimal mannitol concentration for the preservation of potato germplasm[19,20]. This study pioneers the application of the mannitol preservation technique to tillering onions, thereby establishing a novel theoretical framework for the extended preservation of tillering onion germplasm resources.
-
The primary test subjects consisted of the predominant tillering onion variety, Jinlongzhu onion, sourced from Nong'an County in Jilin Province (China). The stem discs of the tillering onion bulbs served as explants for the inoculation process in tissue culture.
Obtaining first-generation tissue culture seedlings
-
The selected explant inoculation materials for the tissue culture experiments were tillering onion bulbs in the sprouting phase, aimed at cultivating primary tissue culture seedlings. Unless stated otherwise, the MS basic culture medium served as the foundation for this study. Each liter of the culture medium was supplemented with 30 g of sucrose and 8 g of agar. The pH was meticulously adjusted to a range of 5.7−5.8 using NaOH and HCl. Before application, the medium underwent sterilization through high temperature and pressure at 121 °C for 20 min. The plant growth hormones and concentrations added during the primary tissue culture seedling cultivation stage were: NAA 0.2 mg/L, IBA 1.2 mg/L.
Screening of growth height of primary tissue culture seedlings and propagation and longitudinal cutting ratio
-
This segment of the study employs a two-factor split-plot design, utilizing DPS software for experimental design and statistical analysis. The primary treatments consist of three distinct plant growth heights: 40, 60, and 80 mm. Secondary processing involves three different longitudinal cut ratios: 1:2, 1:3, and 1:4, as depicted in Fig. 1. Tissue culture seedlings that have been inoculated in the culture vessels and have reached the specified heights of 40, 60, and 80 mm are selected and transferred to a sterile workbench. Following a half-hour UV sterilization, the seedlings are carefully extracted from the vessels and using tweezers and scalpels, the surrounding culture medium is gently removed from the root zone. The upper leaves and lower fibrous roots are excised, and the bulb explants are longitudinally sectioned with a portion of the stem disk according to the three specified ratios: 1:2, 1:3, and 1:4. Each explant is trimmed to a length of 16 mm and subsequently inoculated into the prepared culture medium. According to different cutting ratios, the 1-cut-2 was inoculated into a bottle named T1, the 1-cut-3 was inoculated into a bottle named T2, and the 1-cut-4 was inoculated into a bottle named T3, for a total of nine treatments, each comprising ten explants, replicated three times, culminating in a total of 270 explants.
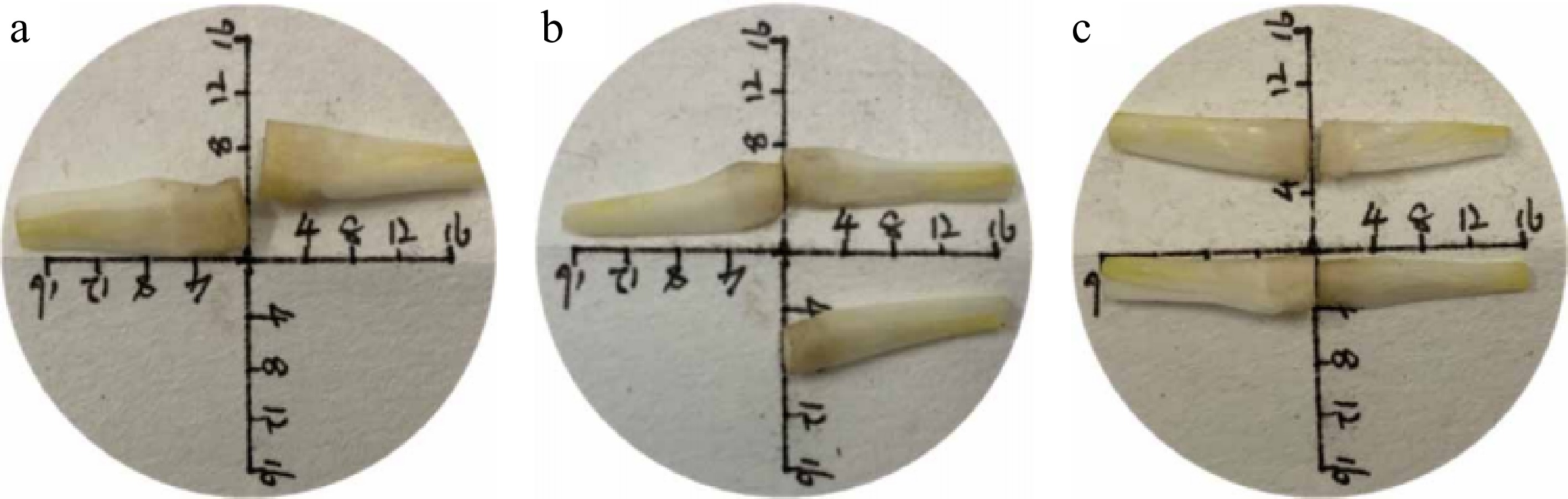
Figure 1.
Schematic diagram of different longitudinal cutting ratios for tillering onions. (a), (b), and (c) represent the scale of longitudinal slices of a tiller onion, which are 1:2, 1:3, and 1:4, respectively. The scale units in the figures are in millimeters (mm).
Screening of optimal mannitol concentration in storage medium
-
Select the optimal height and length-to-cut ratio of tissue culture seedlings identified in the preceding experiment. Place them on a sterile workbench and, after subjecting them to UV sterilization for 30 min, carefully extract them from the culture vessels. Utilize forceps and a scalpel to delicately remove the culture medium surrounding the root systems. Trim the upper leaves and lower fibrous roots of the specimens, and inoculate the longitudinally sectioned bulb explants onto mannitol media at varying concentrations. The MS basic culture medium was used with a hormone ratio of NAA 0.2 mg/L and IBA 1.2 mg/L. The mannitol concentration gradient was set at 2%, 4%, 6%, and 8%, and a blank control (without mannitol) was set. Each treatment was repeated three times, and a total of 300 explants were inoculated.
Measurement indicators and methods
-
Plant height: Natural vertical height from the base of the plant at the top of the medium to the highest point of the plant leaves (ruler measurement).
Pseudostem thickness: Diameter of the thickest part of the pseudostem (vernier caliper).
Root length: Length of the root system (ruler measurement).
Bulb weight: Weight after removing roots, and upper stems and leaves (weighing method).
Bulb diameter: Circumferential length of the enlarged part of the bulb (vernier caliper).
Root length: Average length of all roots of the plant (root scanner).
Number of roots: Number of roots that can be observed (counting method).
Survival rate = Number of surviving plants/Total number of transplants × 100%
Vitrification rate: Ratio of the number of vitrified to the total number of inoculations.
$ \rm{Chlorophyll\;content=(mg/g)(formula\;calculation)} $ (1) $ \rm{R}oot\; vitality:\,=\dfrac{\mathrm{c}}{\mathrm{w}\mathrm{h}}\left[\dfrac{{\text μ}\mathrm{g}}{\mathrm{g}\mathrm{\ \times\ }\mathrm{h}}\right](TTC\; method) $ (2) -
When examining the growth heights of first-generation tissue culture seedlings, notable and highly significant variations in growth morphological parameters were observed across different longitudinal cutting ratios. As illustrated in Table 1, for the first-generation tissue culture seedlings with a growth height of 40 mm, the optimal outcomes for plant height were recorded as follows: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with all three longitudinal cutting ratios exhibiting highly significant differences. Regarding pseudostem thickness, the most favorable results were: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, again demonstrating extremely significant differences among the three ratios. In terms of average plant weight, the best results were: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with highly significant differences noted among the three longitudinal cutting ratios. For leaf count, the optimal effect was: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, where a very significant difference was observed between 1 cut to 2 and the other two ratios, while no significant difference was found between 1 cut to 3 and 1 cut to 4. Lastly, concerning chlorophyll content, the best results were: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with extremely significant differences present among the three longitudinal cutting ratios.
Table 1. Comparison of growth indicators among sub-treatments in the main treatment.
Handle Plant
height (cm)Pseudostem
thickness (mm)Average plant
weight (g)Number of
blades (pcs)Chlorophyll
content (mg/g)Main area Subdistrict 40 mm 1 cut 2 18.33Aa 2.21Aa 0.65Aa 2.65Aa 605.97Aa 1 cut 3 10.94Bb 1.68Bb 0.19Bb 1.98Bb 225.86Bb 1 cut 4 4.94Cc 1.10cc 0.12Cc 1.67Bb 84.95Cc 60 mm 1 cut 2 17.30Aa 1.88Aa 0.70Aa 4.00Aa 763.97Bb 1 cut 3 11.53Bb 1.39Bb 0.56Bb 3.00Ab 688.95Cc 1 cut 4 5.37Cc 1.12Cc 0.23Cc 2.00Bc 906.57Aa 80 mm 1 cut 2 0.41Aa 2.29Aa 0.64Aa 5.00Aa 790.42Bb 1 cut 3 16.77Aa 1.87Bb 0.57Aab 4.00ABb 1013.26Aa 1 cut 4 14.44Cc 1.45Cc 0.54Ab 3.00Bc 783.04Bb Capital letters represent the 1% significant level, and lowercase letters represent the 5% significant level.1 Cut 2 is named T1 in the same cultivation bottle, named T2, 1 cut 3, and 1 cut 4 named T3. In the evaluation of first-generation tissue culture seedlings measuring 60 mm in height, the findings regarding plant height indicated the following hierarchy: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with highly significant differences observed among the three longitudinal cutting ratios. Concerning pseudostem thickness, the optimal results were similarly ranked as: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, again demonstrating highly significant differences across the three ratios. For average plant weight, the results followed the same trend: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with extremely significant differences noted among the longitudinal cutting ratios. In terms of leaf count, the most favorable outcome was: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, where a very significant difference was found between 1 cut to 4 and the other two ratios, alongside a significant difference between 1 cut to 2 and 1 cut to 3. Regarding chlorophyll content, the best results were observed in the order of: 1 cut to 4 > 1 cut to 3 > 1 cut to 2, with extremely significant differences present among the three longitudinal cutting ratios.
In the evaluation of first-generation tissue culture seedlings with a growth height of 80 mm, the findings regarding plant height indicated that the optimal results were observed in the following order: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with highly significant differences noted among the three longitudinal cutting ratios. Regarding pseudostem thickness, the most favorable outcomes were also recorded as: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, again demonstrating highly significant differences among the three ratios. For average plant weight, the most effective ratio was: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, where no significant differences were found between the three longitudinal cutting ratios, nor between the 1 cut to 3 ratio and the other two ratios. Concerning leaf count, the best results were: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with a very significant difference between 1 cut to 2 and 1 cut to 3, while no extremely significant differences were observed between 1 cut to 3 and either 1 cut to 3 or 1 cut to 4. In terms of chlorophyll content, the optimal results were: 1 cut to 3 > 1 cut to 2 > 1 cut to 4, with a very significant difference between 1 cut to 3 and the other two longitudinal cutting ratios, although no significant difference was found between 1 cut to 2 and 1 cut to 4.
At the same time, the growth height of the seedling seedlings in the initial group is not at the same time, and the development indicators of different longitudinal cuts are extremely significant (Table 2).
Table 2. Comparison of developmental indicators among sub-treatments in the main treatment.
Handle Bulb weight
(g)Bulb diameter
(mm)Root length
(mm)Root coefficient
amount (pcs)Root vitality
(μgTTCg−1FW)Main area Subdistrict 40 mm 1 cut 2 0.07Aa 3.25Aa 25.42Aa 7.78Aa 113.19Bb 1 cut 3 − − 15.75Bb 3.79Bb 144.38Aa 1 cut 4 − − − − − 60 mm 1 cut 2 0.22Aa 4.68Aa 59.93Aa 24.79Aa 74.50Aa 1 cut 3 0.10Bb 3.52Bb 44.19Bb 16.12Bb 33.74Cc 1 cut 4 0.05Cc 3.34Bc 38.86Cc 11.12Cc 56.63Bb 80 mm 1 cut 2 0.43Aa 5.61Aa 65.86Aa 26.78Aa 139.40Cc 1 cut 3 0.31Ab 5.06Bb 60.00Bb 23.78Bb 212.18Aa 1 cut 4 0.29Ab 5.29Cc 50.18Cc 20.12Cc 187.64Cc Capital letters represent the 1% significant level, and lowercase letters represent the 5% significant level. 1 Cut 2 is named T1 in the same cultivation bottle, named T2, 1 cut 3, and 1 cut 4 named T3. In the evaluation of primary tissue culture seedlings measuring 40 mm in height, the optimal impact on bulb weight was observed with the treatment 1 cut 2, which outperformed both 1 cut 3 and 1 cut 4, with highly significant differences noted among these treatments. Regarding bulb diameter, the most favorable outcome was also recorded for 1 cut 2, surpassing 1 cut 3 and 1 cut 4, with extremely significant differences evident between these treatments. For root length, the most effective treatment was 1 cut 2, followed by 1 cut 3 and then 1 cut 4, with substantial differences among the three longitudinal cut ratios. In terms of root quantity, the best results were again seen with 1 cut 2, exceeding both 1 cut 3 and 1 cut 4, and significant differences were found among the three longitudinal cut ratios. Lastly, concerning root vitality, the most pronounced effect was achieved with 1 cut 2, which was superior to 1 cut 3 and 1 cut 4, with highly significant differences among the three longitudinal cut ratios.
When the growth height reaches 60 mm, the optimal bulb weight effect of the first-generation tissue culture seedlings is observed in the order of 1:2 > 1:3 > 1:4, with highly significant differences among the three longitudinal cut ratios. Regarding bulb diameter, the most favorable outcome is also 1:2 > 1:3 > 1:4, where a very significant difference exists between the 1:2 ratio and the other two ratios, as well as a significant difference between the 1:3 and 1:4 ratios. In terms of root length, the optimal effect follows the same pattern: 1:2 > 1:3 > 1:4, with highly significant differences among the three longitudinal cut ratios. For the number of roots, the best effect is again 1:2 > 1:3 > 1:4, demonstrating extremely significant differences among the three longitudinal cut ratios. Lastly, in terms of root vitality, the optimal effect is ranked as 1:2 > 1:4 > 1:3, with extremely significant differences among the three longitudinal cut ratios.
In the evaluation of the first generation of tissue culture seedlings, which exhibited a growth height of 80 mm, the analysis of bulb weight revealed the following hierarchy: 1 cut to 2 > 1 cut to 3 = 1 cut to 4. A significant difference was observed between the 1 cut to 2 ratio and the other two ratios, while no significant difference was noted between the 1 cut to 3 and 1 cut to 4 ratios. Regarding bulb diameter, the optimal results were: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, with extremely significant differences among the three longitudinal cut ratios. For root length, the best performance was also: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, demonstrating a very significant difference across the three longitudinal cut ratios. In terms of root quantity, the most favorable outcome was: 1 cut to 2 > 1 cut to 3 > 1 cut to 4, again showing a very significant difference among the three longitudinal cut ratios. Lastly, concerning root activity, the best effect was observed in the ratio of 1 cut to 3 > 1 cut to 2 = 1 cut to 4, with a very significant difference between 1 cut to 2 and 1 cut to 3, while no significant difference was found between 1 cut to 2 and 1 cut to 4. However, a very significant difference was noted between 1 cut to 3 and 1 cut to 4.
Conduct principal component analysis on various indicators measured in tillering onion tissue culture seedlings
-
The most critical indicators for assessing the height and longitudinal cut ratio of primary tissue culture seedlings should be selected. Based on the analytical results presented in Table 3, it is evident that principal component 1 is the most significant factor in elucidating the ten measurement indicators of onion tissue culture seedlings, exhibiting a variance contribution rate of 65.761%. Within principal component 1, the loadings for the majority of traits were notably high, particularly for bulb diameter, root length, and root number, all of which surpassed the loading threshold of 0.9, thereby underscoring their substantial contribution to principal component 1. The variance contribution rates for principal component 2 and principal component 3 are 12.400% and 10.873%, respectively, resulting in a cumulative contribution rate of 89.033%, which accounts for the majority of the data variation. Plant height demonstrates a higher loading on principal component 2, whereas stem diameter exhibits a higher loading on principal component 3, indicating that these two traits significantly contribute to their respective principal components.
Table 3. Principal component eigen vectors and cumulative contribution rates of ten determination indicators of tillering onion tissue culture seedlings.
Project Characters Principal component 1 Principal component 2 Principal component 3 Eigen value 6.576 1.24 1.087 Variance contribution rate (%) 65.761 12.4 10.873 Cumulative contribution rate (%) 65.761 78.16 89.033 Eigen vectors Plant height 0.466 0.835 0.013 Stem thickness 0.625 −0.221 0.665 Average weight per plant 0.813 −0.311 0.013 Number of blades 0.815 −0.116 0.324 Chlorophyll content 0.835 0.223 −0.449 Weight of bulb 0.918 −0.114 0.101 Diameter of bulb 0.954 −0.055 −0.256 Length of roots 0.951 −0.082 −0.245 Number of roots 0.938 −0.208 −0.205 In conclusion, bulb diameter, root length, and root count have been identified as the key parameters for evaluating the growth height and longitudinal cut ratio of primary tissue culture seedlings, with plant height and stem diameter being significant secondary factors. Utilizing these five essential metrics, we will assess the growth height and longitudinal cut ratio of the first-generation tissue culture seedlings.
Variance analysis on the main and secondary factors of tillering onion tissue culture seedlings
-
The analysis presented in Table 4 regarding the variance of morphological growth parameters between the treatments of growth height and longitudinal cut ratio for the first-generation tissue culture seedlings of tillering onion reveals noteworthy findings. It is evident that significant differences were observed in the expanded seedling height, pseudostem diameter, average individual plant weight, leaf count, and chlorophyll content between the growth height and longitudinal cut ratio treatments, reaching levels of extreme significance. Furthermore, in the interaction between the growth height of the first-generation tissue culture seedlings and the longitudinal cut ratio, while no significant differences were noted in plant height and pseudostem diameter, a marked difference was observed in leaf count. Both average plant weight and chlorophyll content exhibited highly significant variations.
Table 4. Variance analysis table of morphological growth indicators between main treatment and secondary treatment.
Handle Factor Plant height
(cm)Pseudostem
thickness (mm)Average plant
weight (g)Number of
blades (pcs)Chlorophyll content
(mg/g)Main area Factor A 26.45** 28.76** 9.01** 19.91** 177** Subdistrict Factor B 26.31** 156.41** 10.97** 8.58** 29787.88** AB interaction A × B 1.21 2.87 4.76** 3.29* 105874.2** ** Indicates 1% extremely significant level, * indicates 5% significant level. In an investigation aimed at understanding the factors influencing the subsequent propagation of tillering onion tissue culture seedlings, we employed a two-factor split-plot experimental design to assess the impacts of varying growth heights and longitudinal cutting ratios of the first-generation tissue culture seedlings. The findings indicated that regarding the growth height of the first-generation tissue culture seedlings, those at a height of 80 mm exhibited superior performance across five key parameters: plant height, pseudostem thickness, bulb diameter, root length, and root count. Additionally, concerning the longitudinal cutting ratio, the tissue culture seedlings subjected to a ratio of 1:2 also demonstrated optimal growth performance in these parameters.
Explore the optimal mannitol concentration in the preservation medium
Growth morphology indicators at different mannitol concentrations during the same period
-
In an experiment aimed at identifying the optimal mannitol concentration for preservation media, we observed from the data in Table 5 that tissue culture seedlings exhibited the most robust growth at a concentration of 0% mannitol. As the concentration increased, various physiological parameters showed a gradual decline, with an 8% concentration notably inhibiting growth. The most pronounced effect was a significant reduction in the growth rate, while a 4% concentration resulted in the most severe vitrification phenomenon. Following our screening process, we established that 2% and 8% mannitol concentrations were optimal for the preservation medium, leading to further subculturing for additional screening and validation.
Table 5. Comparison of various indicators of tissue culture seedlings under different concentrations of mannitol treatment over time.
Handle Plant height
(cm)Root length
(cm)Root coefficient
amount (piece)Pseudostem
thickness (cm)Vitrification
(%)Time Mannitol concentration 15 d 0% 3.88Aa 1.63Aa 1.26Aa 1.19Aa 0Aa 2% 2.78Bb 1.05Bb 0.67Bb 1.07Aa 0Aa 4% 2.40BCb 0.96Bb 0.65Bb 0.98Aa 0Aa 6% 1.96Cc 0.93Bb 0.60BCb 0.94Aa 0Aa 8% 1.04Dd 0.91Bb 0.41Cc 0.92Aa 0Aa 30 d 0% 6.44Aa 2.76ABa 1.68Aa 1.39Aa 0Dd 2% 4.63Bb 2.36BCb 0.87Bb 1.02ABb 0Dd 4% 3.33Cc 2.92Aa 0.28Cd 0.74Bb 2.65Aa 6% 2.64Dd 2.37BCb 0.65BCbc 1.04ABb 1.74Bb 8% 1.13Ee 2.24Cb 0.37Ccd 0.78Bb 0.51Cc 45 d 0% 9.03Aa 3.41Aba 2.97Aa 1.64Aa 0.34Cd 2% 6.28Bb 2.88Bb 2.05Bb 1.81Aa 0.11Cd 4% 5.58Cc 3.68Aa 1.00Cc 1.45Aa 3.10Aa 6% 3.86Dd 3.27ABab 0.97Cc 1.69Aa 1.73Bb 8% 1.63Ee 2.88Bb 1.26Cc 1.79Aa 0.65Cc Capital letters represent the 1% significant level, and lowercase letters represent the 5% significant level. Growth indicators of primary tissue culture seedlings after transfer culture
-
The data presented in Fig. 2 indicates that the growth and survival rates of tissue culture seedlings preserved in mannitol medium and subjected to subculturing exhibit highly significant variations across different mannitol concentrations. Notably, tissue culture seedlings maintained at a 2% mannitol concentration demonstrate plant heights that closely approximate those of the control group, with statistically significant differences observed, and their survival rate is the highest among all tested concentrations, achieving rates of 4%, 6%, and 8%. At this concentration, the performance of the histocultured seedlings was unparalleled, exhibiting exceptional growth conditions, as we can also observe in conjunction with Fig. 3. In contrast, the plant heights at 4% and 6% concentrations reveal significant differences when compared to the control group, with survival rates falling below that of the 2% mannitol concentration. Furthermore, the plant height at the 8% concentration is the lowest, exhibiting the slowest growth rate and a survival rate of only 74%, which is detrimental to the subculturing of tissue culture seedlings.
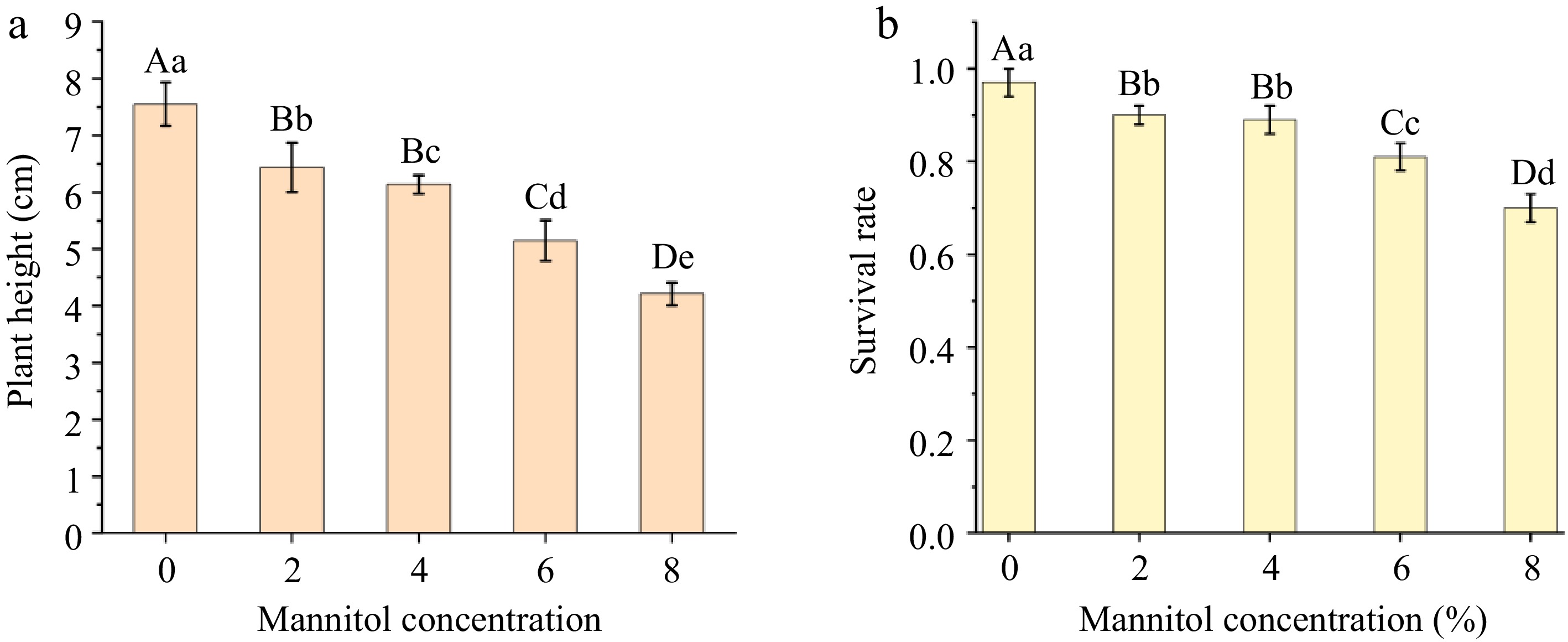
Figure 2.
Multiple comparisons of plant height or survival rate of tissue culture seedlings under different mannitol concentrations. Capital letters denote the 1% significance level, while lowercase letters indicate the 5% significance level. (a) Illustrates multiple comparisons of plant height among plant tissue culture seedlings at various mannitol concentrations. (b) Depicts multiple comparisons of survival rates among plant tissue culture seedlings at different mannitol concentrations.

Figure 3.
Comparison of growth conditions at different mannitol concentrations. The figure shows the growth of explants inoculated on July 11th and September 26th, and observed 45 d later at five concentrations of 0% (CK), 2%, 4%, 6%, and 8% mannitol.
Screening of optimal mannitol concentration
-
By evaluating the growth parameters associated with varying concentrations of mannitol in the preservation medium, we identified the concentrations that exhibited the lowest growth rates, maintained normal growth conditions, minimized vitrification, and demonstrated reduced vitrification rates as 2% and 8%. Following subculturing and transfer culture, we further refined our selection to a mannitol concentration of 2%. The tissue culture seedlings at this concentration displayed normal growth characteristics, with plant heights comparable to the control group, and achieved the highest survival rate. Consequently, a 2% mannitol concentration is deemed more appropriate for the preservation and transfer of tillering onion explants.
-
In the group cultivation of seedlings, the addition of glycol reduces cell swelling, increases the infiltration of the medium, obstructs the absorption of moisture and nutrients, and weakens metabolic activities, thereby extending the secondary time[21]. In this study, 20g/L glycolol was added. Under the premise of high survival rate of seedling seedlings, the effect of saving and transmiting the onion is the best, which can effectively extend the saving time. This has been studied with Li et al.[20] and others. The concentration of the most suitable mannitol for potatoes is the same.
The group cultivation seedlings after the extension preservation test can be cultivated under normal conditions, which can restore growth and restore its biological characteristics and physiological activity before preservation[22]. The activity of enzymes such as features, SPAD values, POD, and CAT gradually approaches CK in terms of value, indicating that the split onion group cultivation after the savory ionizing has maintained its genetic stability, and the method of saving from the body can be feasible.
In the evaluation of mannitol-based preservation media, following the initial generation of tissue culture seedlings at varying mannitol concentrations, it is evident that the limited measurement duration necessitates further observation and validation of the specific preservation period. The vitrification phenomenon is most pronounced at a 4% mannitol concentration. It remains to be investigated whether the vitrification of tillering onion tissue culture seedlings can be effectively mitigated by reducing the concentrations of cytokinins and gibberellins while simultaneously increasing the agar concentration, warranting further in-depth exploration. Research conducted by Huang et al. indicated that during the mannitol preservation of sweet potatoes and subsequent field planting, there exists a negative correlation between mannitol concentration and yield[23]. It is imperative to determine if a similar correlation is present in the preservation of tillering onions and whether it impacts subsequent field planting or commercial seedling production, necessitating further testing and validation in future studies.
-
The experiment demonstrated that the propagation efficacy of first-generation tissue culture seedlings is maximized at a height of 60 mm, with an optimal longitudinal cutting ratio of 1:2. Additionally, during the evaluation of mannitol preservation media, a concentration of 2% mannitol yielded the most favorable results, while a 4% concentration resulted in the most pronounced vitrification phenomenon.
This study was funded by the Jilin Provincial Tiller Onion Germplasm Resources Innovation Team Project (Grant No. 20240601066RC).The authors are grateful to the Jilin Agricultural University and the Tiller Onion Germplasm Resources Innovation Team for providing facilities to carry out the present study and to perform the chemical analyses.
-
The authors confirm contributions to the paper as follows: research conception and design: Wang H, Chen H; data collection: Chen H, Wang Q, Gao X; analysis and interpretation of results: Wang H, Wang Q; preparation of draft manuscript: Chen H, Gao X; supervision: Qiao H, Zhang J. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Huiying Wang, Huijie Chen
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang H, Chen H, Wang Q, Gao X, Zhang J, et al. 2025. Optimization of subculture and preservation system of tillering onion tissue culture seedlings. Technology in Horticulture 5: e017 doi: 10.48130/tihort-0025-0015
Optimization of subculture and preservation system of tillering onion tissue culture seedlings
- Received: 15 December 2024
- Revised: 26 January 2025
- Accepted: 11 March 2025
- Published online: 01 May 2025
Abstract: Tillering onion (A. cepa L. var. aggregatum G. Don.) represents a specific variant of onion. Prolonged reliance on bulbs as the primary reproductive organ in the cultivation of tillering onions has led to significant degeneration of the species, resulting in marked declines in both yield and quality. Additionally, the use of test tube seedlings for direct propagation eliminates the necessity for bulb formation. By directly domesticating and transplanting these propagated seedlings into the field, a substantial quantity of seedlings can be produced in a relatively short timeframe. To study the optimal subculture system for tiller onions, this research utilized first-generation tissue culture seedlings as experimental subjects. Various longitudinal cutting ratios and growth heights were implemented, and the results indicated that for 80 mm first-generation tissue culture seedlings, the optimal longitudinal cutting ratio was 1 to 2, with all principal component analysis indices being optimal. Furthermore, the study assessed the preservation conditions at different mannitol concentrations. The findings revealed that the best preservation, slowest growth, and lowest vitrification were obtained at a 2% mannitol concentration, with a survival rate of up to 98% for the successor culture. This study aimed to optimize the successional culture system of tiller onion bulb disc tissue and to lay the foundation for the commercial cultivation of tiller onion bulbs and in vitro conservation of germplasm resources.
-
Key words:
- Tillered onions /
- Subculture /
- Slitting ratio /
- Growth height /
- Mannitol












