-
Sunlight contains some ultraviolet (UV) light that is harmful to living things, but it does not cause excessive harm to living things on Earth due to the ozone layer which absorbs these UV light emissions. With the global greenhouse effect and increasing air pollution over recent years, chemicals such as chlorofluorocarbons have increased in the atmosphere. This phenomenon depletes the ozone layer, which results in its thinning. For every 1% depletion of the ozone layer, harmful UV light reaching the Earth's surface increases by 2%, which will eventually lead to a significant increase in UV radiation on the surface of the Earth[1].
UV irradiation is a universal environmental stress factor that can affect all organisms. Strong UV radiation can exert a strong selection pressure on insects, change the enzyme activity in insects, and even alter the genetic material of insects, which results in genetic differentiation. Insects can be extremely sensitive to micro changes in the surrounding environment and make timely changes. These environmental changes will cause the destruction of homeostasis in the insect body and force corresponding physiological and biochemical changes. Insects, as the oldest and most abundant biological group in nature, have evolved a special defense system to maintain insect homeostasis for adapting to the toxicity of host plants and the stress of adverse living environments in the long-term process of biological evolution[2]. Insects have various adaptive enzymes that respond to external stress, such as heat shock proteins (Hsps), detoxification enzymes, and protective enzymes[3]. These adaptive enzymes play distinct roles. For instance, Hsps is essential for insect resistance to temperature stress, detoxification enzymes are crucial for insect resistance to plant secondary substances and toxins, and protective enzymes are responsible for removing reactive oxygen species from insects exposed to UV light stress, CO2 stress, and other stressors. Protective enzymes and detoxification enzymes are the primary mechanisms in insects to cope with UV stress[4]. According to Zhang et al., detoxification enzymes and protective enzymes are considered the primary adaptive proteins involved in insect defense mechanisms[5], which play an important role in various adaptive pathways such as avoidance of feeding, detoxification metabolism, and resistance to adverse external environments[6]. These systems are often used as a measure of changes in the physiological state of of insects[7].
The activity of insect detoxification and protective enzymes can be induced by harmful substances or stress, and the enzymes in the body can quickly respond to adverse environmental stress to ensure that the insects can adapt to the environment. Under the induction of toxic secondary substances produced under UV light stress, the detoxification and protective enzyme systems in the insect body are activated, and their activities are also changed to remove or decompose toxic substances in the body for protecting insects from harm. At present, relevant studies on the changes in insect detoxification and protective enzyme systems under UV light stress are being conducted. For instance, Li et al. investigated the impact of varying intensities of ultraviolet light on the ecological characteristics and enzyme activities of aphids[8]. Tian examined the influence of UV-B radiation on the growth, development, and antioxidant enzyme activity of Neoseiulus barkeri[9], which showed that long-term UV irradiation can cause direct or indirect damage to the insect body. Severe oxidation stress can change the physiological function of enzyme systems in vivo and accelerate the oxidation process of proteins, and it can even lead to protein denaturation and inactivation[10].
The effect of UV on insects and the phototaxis or photophobism of insects has always been an important means of green control technology for plant protection. Control measures based on this technology, such as black light and UV light, have been widely used in integrated pest management[11]. In this study, the effects of UV light on insect physiology and biochemistry, the activity changes in detoxification and protective enzymes under UV light stress, and the adaptation mechanism of insects enzymes responding to UV light stress were reviewed. This review is helpful for readers to know and understand the physiological countermeasures of insects to cope with UV light, in order to provide a more theoretical basis for enriching and improving the application of UV comprehensive pest control and promote the development of the physical pest control industry.
-
UV radiation are harmful electromagnetic waves with a wavelength smaller than visible light. Most of the UV light on the Earth's surface comes from the sun, and the harmful effects of sunlight on biological systems are nearly entirely caused by the radiation in the UV spectrum emitted by the sun[12]. UV light is divided into three bands, according to the length of its wavelength: UV-A (400-320 nm), UV-B (320-280 nm), UV-C (280-100 nm)[13]. The damage to biological bodies is greater when the wavelength of UV light is shorter. However, given that UV wavelengths less than 290 nm can be absorbed by the ozone layer of the Earth's atmosphere, more than 90% of the UV reaching the ground is in the UV-A band[14].
UV is an essential part of the life activities of insects, and many insect behaviors are regulated by UV under normal circumstances[15]. The intensity of UV radiation will change regularly with the alteration in the altitude angle of the sun at different times, and this change will cause variations in the visual protein of insects. An increasing number of studies have confirmed that many insects are sensitive to UV-A light. Cronin & Bok found that UV vision is vital for insects' color perception, allowing them to accurately perceive subtle variations in the complex spectral information of their surroundings[16]. This ability arises from insects' ability to perceive UV light as a primary color, facilitated by the visual pigment composed of opsin and chromophore in their photoreceptors. Insects receive UV light signals through the integration of visual information from the UV-sensitive opsin (UVRh) and other opsins in the central nervous system[17].
A growing body of research supports the idea that UV radiation has a significant impact on the behavioral traits of insects. Insects have the ability to detect and respond to UV light, mainly because they contain UV opsin[18]. For example, Caliothrips phaseoli has been observed to be highly attracted to UV-B radiation in the 290-330 nm range[19]. Plants display various responses to UV radiation, such as a decrease in phloem cell count and an increase in the thickness of the cuticular wax layer. Moreover, exposure to UV light prompts plants to produce secondary metabolites, like phenolic compounds, that hinder insect feeding. These changes undoubtedly affect the feeding behavior of herbivorous insects[20]. Briscoe & Chittka found that UV-A radiation affected the insect search for food, flight, location, mate selection, and the interaction between the sexes[21], which would influence the geographic distribution and range of insect populations[22]. The results of studies on B. tabaci have shown that insufficient UV exposure disrupts the insect's ability to locate its flight, thereby altering its reproductive and dispersal behavior[23]. Some insects need UV-B to find a suitable habitat[14]. The effects of UV on insects are summarized in Table 1. These data all show that UV radiation greatly impacts insects.
Table 1. Effects of UV stress on biological characteristics of insects.
Biological effect Insect Biological effects of UV on insects Behavioral characteristics Phototactic behaviour Diaphorina citri It is taxis to a variety of wavelengths of monochromatic light including 360 nm UV[34]. Drosophila melanogaster Low intensity UV produces a momentary attraction to D. melanogaster, while high intensity UV causes avoidance[35]. Trophic behaviour Bombus friseanus Red flowers can stimulate the photoreceptors of B. friseanus by reflecting UV, which makes it inclined to choose red flowers for food[36]. Dispersal behavior Bemisia tabaci The flight localization ability of whitefly would be destroyed under insufficient UV irradiation, and then, the propagation and diffusion behavior of whitefly would be changed[15]. Courtship behavior Bicyclus anynana The male is able to select a mate based on the eye-shaped markings on the female's wings that reflect UV[37]. Pieris rapae In the UV-light abundant environment, 96.20 % female butterflies showed active courtship behavior, and the mating success rate was 37.33 %, which was significantly higher than that in the UV-light deficient environment[38]. Parasitic behavior Corcyra cephalonica It was found that the parasitism rate of egg Trichogrammatid irradiated by UV light was the highest[39]. Avoid natural enemies Lopinga achine When the light intensity is low and most of it is UV, it can avoid deadly attacks from aerial enemies[40]. Biological characteristics Growth and development Macrosiphum avenae Inhibited the molting of nymphs, prolonged the development period, and caused population degradation[41]. Myzus persicae Damaged dermal cells and significantly affected the relative expression of CHS1[26]. Prolonged development time and average generation cycle, which stimulated fertility[16]. Acyrthosiphon pisum With the extension of radiation time, the number of generations increased, and the body weight and body length first increased and then decreased[42]. Reproduction Dialeurodes citri When a pair of D. citri was irradiated with UVA for 0, 1, 4 and 7 h/d, it was found that the fecundity and spawning capacity of the other treatments were significantly increased compared with the control group except for 7 h/d[43]. Physiological characteristics Enzyme system Myzus persicae The expression of four protein-coding genes (coenzyme NADH, heat shock protein 70, epidermal protein, and REDOX enzyme) was activated, and the transcription level was enhanced[44]. Immune system Bombyx mori Feeding B. moriwith UV-B irradiated mulberry leaves enhanced its resistance to nuclear polyhedrosis virus[45]. Scholars have also conducted relevant studies on the effects of UV on the biological characteristics of insects . Studies have shown that an appropriate amount of UV light can benefit the development of insects, and an appropriate amount of UV light can improve the immune function of insects[24], while an excessive amount of UV light can inhibit the reproduction of insects[25]. Zhou studied the changes in the inherent growth rate, net proliferation rate, and average generation cycle of Sitobion avenae[26]. The author concluded that short-term and low-intensity UV-B was beneficial to the growth and development of insects, and it could prolong the number of spawning days and the total life span of the insects, and vice versa. Long-term UV-B stress can delay the molting time and prolong the development period of Myxaphis nymphs, which suggests obvious population degradation. The effect is more significant and the survival rate and fertility is lower when the generation under UV-B stress is longer, and long-term UV-B irradiation can also destroy dermal cells[27].
Under normal conditions, UV radiation-induced changes in the external morphology of insects typically manifest in the offspring of UV-irradiated parents, resulting in a small proportion of deformed individuals[28]. Extensive data demonstrates that intense UV-B radiation can directly or indirectly harm insects and cause genetic variations, resulting in DNA breakage, base mutation, or deletion, ultimately altering the genetic stability of organisms[29]. Prolonged exposure to intense UV-B radiation exerts strong selection pressure on insects, leading to subspecies genetic differentiation. Moreover, after being exposed to UV radiation, there was an increased proportion of S. avenae in the offspring, which enhanced their ability to evade UV-induced stress and relocate to a more favorable environment for survival[30].
UV radiation significantly affects the physiological characteristics of insects. Specifically, when insects are exposed to UV radiation, their DNA bases have the ability to absorb the energy emitted by UV-B photons, resulting in the formation of photooxidation products[31]. Consequently, when these oxidized bases are involved in DNA replication, the probability of mismatches increases significantly, hindering the transmission process and ultimately compromising the structural integrity of insect genetic material[32]. Exposure of insects to UV radiation for multiple generations leads to varying degrees of changes in the protective enzymes associated with antioxidant and detoxification functions in their bodies, as well as the enzyme activity of detoxification enzymes[33].
-
In the long process of coevolution between plants and insects, plants have formed many physical and chemical defense mechanisms through morphology, biochemistry, and molecular regulation to protect themselves from insects. In addition to facing the stress of plant toxic secondary substances and foreign harmful substances, insects will encounter stress such as high temperature, high pressure, strong UV light, high concentration of CO2, and other adversities. When insects are in a stressful environment, the detoxification and protective enzymes in their bodies can cooperate to make corresponding feedback regulations quickly; the activity of the enzyme system also changes significantly, which helps insects adapt to certain environmental changes and improve their survival ability[46].
Insect detoxification enzymes are a class of heterogeneous enzyme systems mainly present in the midgut, martensitic tube, microsome, mitochondria, adipose bodies, and Golgi bodies of insects, which can metabolize and decompose a large number of endogenous or exogenous toxins in insects. The function of the detoxification enzyme series is to transform lipophilic foreign toxic compounds into hydrophilic nontoxic products by binding with sugar, amino acid, or sulfate groups, which enhances their dissolution and excretion rate in the body. Detoxification enzymes mainly include cytochrome P450 (CYP450), glutathione S-transferases (GSTs) and esterase (EST). The toxic compounds metabolized by insects into the body mainly include three stages: the first stage is that CYP450 and esterase introduce or release the functional group of harmful substances and improve the water solubility of harmful substances, and the period easily becomes hydrophilic. The second stage is that GSTs will further detoxify the processed activated harmful substances to form conjugates and will reduce their lipophilicity, which results in the loss of the ability of harmful substances to penetrate the cell membrane for reducing or eliminating the toxicity of toxic substances. The third stage is that the ABC transporter transports the toxin conjugates across the membrane to the outside of the cell[47].
In addition to the detoxification enzyme system that degrades foreign harmful substances, insects have superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) systems and other protective enzyme systems[48]. Under normal circumstances, free radicals can maintain the normal physiological functions of the matrix. However, when insects are under adverse conditions, the production rate of free radicals in the cells in the body will be greater than the removal rate, which causes the accumulation of reactive oxygen species (ROS) in the body. These free radicals have a strong oxidizing ability on the insect body, which will lead to lipid peroxidation of insect cell membranes, DNA double strand break, base mutation, and protein structure destruction and thus cause serious damage to cell function. The main function of protective enzymes is to remove free radicals in insects and prevent the production of a large number of ROS in the body from causing damage[5].
-
Metabolic detoxification mediated by detoxification enzymes is one of the regulatory methods of insect response to UV stress. Cyp450-mediated multifunctional oxidase is the main detoxification enzyme system that regulates detoxification metabolism in insects[49]. It is named as such because its binding with CO has the maximum light absorption value at 450 nm when it is in the reducing state. This enzyme system exists in nearly all the oxidase systems in the body[50]. CYP450, as the terminal oxidase of the entire enzyme system, determines the specificity and diversity of the substrate of the entire enzyme system due to its structural complexity and variety[51]. CYP450 must form a multienzyme complex structure with a series of related enzyme proteins to perform its oxidation function[52]. More than 300 CYP450 species have been identified in insects, and they are distributed in 48 CYP450 families[53].
Meng found that 29 proteins of Aphis gossypii Glover adults were significantly affected after exposure to UV-A radiation, and most of these changes were related to stress response[54]. Therefore, some scholars speculate that the activation of the stress response mechanism may play an important role in reducing the negative effects of UV light exposure on insects. Some scholars have explored the stress response genes of the CYP450 family to verify the aforementioned hypothesis. Sang et al. studied the response of the CYP450 gene in Tribolium castaneum exposed to UV-A. UV-A can induce the expression of CYP6BQ4 and CYP6BQ8, while it can significantly inhibit the expression of CYP6BQ11[55]. Although the expression profiles of P450 family genes in these insects were affected by UV-A, this effect did not cause serious negative ecological damage to insects. Ten potential TFBMS that may be involved in the co-expression regulation response to UV-A stress, were found in the upstream sequence of upregulated CYP450. The same has been found in mammalian cells, and these genes may help protect organisms against UV-induced damage[56].
CarE and cholinesterase (ChE) are the main detoxification metabolites in esterase. CarE, as an important part of insect chemical defense system, can be related to detoxification metabolism of organophosphorus insecticides and other drugs, and its main function is to hydrolyze toxic compounds containing ester bonds[57]. The distribution of CarE is different in different body segments of insects and mainly concentrates in the head and chest[58,59]. Studies have shown that the activity of CarE in insects was lower than that in the control group when UV radiation was applied for 1.5 h, while the activity of CarE in insects began to be significantly upregulated after UV stress for 2 h, which indicates that CarE can help insects adapt to UV stress[54]. Meng et al. found that AChE and CarE activities in Helicoverpa armigera was inhibited to varying degrees, and the inhibition degree of enzyme activity was the highest at 30 min, which was significantly lower than the control level[60].
GST and protective enzymes such as SOD, CAT, and POD and glutathione reductase, thioredoxin reductase, and others together constitute the main antioxidant enzyme systems of insects. GST is a major detoxification enzyme that regulates glutathione metabolism in the antioxidant systems. Glutathione peroxidase (GPOx) is dependent on selenocysteine not being present in insects, and GST can replace GPOx in removing lipid peroxides in insect cells. However, GST cannot clear H2O2 in cells[61]. UV-B radiation can significantly affect the GST activity of insects. Studies have found that GST in the first generation of S. avenae irradiated with UV-B is inhibited, but GST activity will gradually increase with the rise in insect generations under UV-B irradiation, and GST activity will also improve with the enhancement in UV radiation intensity[33]. Zheng treated male and female D. melanogaster under different UV-A intensities and irradiation times, and the results showed that UV-A stress led to the increase in GST activity[62]. Moreover, differences were found in the changes in GST activity between male and female insects of the same group under UV-A stress. The findings are consistent with the results of Wang et al. on the effects of antioxidant detoxification enzymes on Dendrolimus tabulaeformis under UV-A radiation[63]. The reason for this difference may be due to the fact that females are responsible for reproduction, which requires them to bear more pressure from the external environment.
Effects of UV light stress on protective enzyme systems
-
SOD are one of the fastest catalytic enzymes in the known enzymes, and it mainly catalyzes the decomposition of superoxide anion free radicals (O2−) to produce O2 and H2O2; it is one of only a few enzymes in insects that can eliminate superoxide anion free radicals, and it is also the first line of defense in insects to prevent damage caused by free radicals[64]. POD is a bio-terminal oxidoreductase, which not only can catalyze the transformation of H2O2 into quinones to eliminate H2O2 and decompose harmful substances generated by the aerobic dehydrogenation metabolism of organisms, but can also constitute an important component of the anti-lipid peroxidase protection system in cells[25]. CAT has been proven to exist widely in various organisms and is a class of antioxidants. CAT is a binding enzyme with iron porphyrin as a prosthetic group, and a porphyrin heme group is present at the active site of each subunit to catalyze the decomposition of H2O2 into O2 and H2O for removing H2O2 in insects. The mechanism by which CAT acts on H2O2 is essentially the disproportionation of H2O2, and the catalytic reaction can only occur when two H2O2 meets CAT successively and collide on the active center[65]. SOD and CAT are common in insects, but a few insects do not contain POD.
Li et al. found that the activities of SOD, POD, and CAT of insects under UV-A and UV-B irradiation increased with the extension of light time, and the activities were significantly greater than those in the visible light treatment group, in which UV-B had a greater effect on the activities of the three protective enzymes[66]. Meng et al. tested the effects of different UV irradiation times on three protective enzymes of H. armigera Hubner, and the activities of SOD, POD, and CAT were significantly increased after 30 min of UV irradiation[10]. Li et al. found that the activities of CAT, POD, and SOD in S. avenae after UV-B stress were significantly higher than those in normal insects[8]. Under long-term UV-B stress, the activities of CAT and POD decreased significantly, while the activities of SOD increased significantly. The results showed that SOD played a dominant role in the three protective enzymes during the resistance of A. pisum to UV-B radiation. The study also found that the red type of Acyrthosiphonpisum had stronger resistance to UV stress than the green type[41]. Ali et al. found in Spodoptera litura that GST activity increased significantly and antioxidant capacity rose after 60 and 90 min exposure to UV-A[67]. GST activity and total antioxidant capacity decreased significantly after 120 min exposure, and longer exposure time reduced their ability to resist ROS. This result is consistent with the findings of Karthi et al. on the effects of UV-B-induced oxidative stress on the antioxidant response of Spodoptera litura[68]. Although UV stress can promote the increase in protective enzyme activity, this promotion effect cannot exceed the maximum tolerance limit of insects, and long-term or high-intensity UV radiation is ultimately harmful to insects[69].
-
Insects exposed to long-term UV stress have developed corresponding adaptive mechanisms in behavior, structure, and physiology throughout the course of evolution. Behavioral adaptations in insects primarily involve reducing the risk of harm by seeking shelter in shaded areas of plants when exposed to UV radiation. For example, plant-eating insects will avoid damage by heading to the back of the leaf when they are exposed to UV radiation. The UV radiation intensity on the back of the leaf is much lower, which can reduce the risk of harm to insects exposed to UV. Studies have shown that Aphis glycines in the field always accumulate on the back of leaves after exposure to UV radiation. In the study on D. melanogaster, it was found that they avoid ultraviolet light when laying eggs[70]. Structural adaptations include a series of defense mechanisms that alter body structures such as exoskeletons, fur, wings, and photoprotective pigments to resist UV stress. For example, after UV-A irradiation of insects, it is observed that the scaly arrangement of the back epidermis is blurred, a large amount of secretions are attached to the surface of the epidermis, and the outer epidermis becomes thick. In addition, melanin in insects has photoprotective properties and can participate in insect immune response. When insects are exposed to strong UV radiation, the body will resist radiation through the accumulation of melanin[70]. Among all insect adaptations, physiological adaptations relying on protective and detoxifying enzymes are the most crucial, serving as the primary adaptation mechanism under UV radiation. Insects respond to UV radiation by coordinating enzyme systems, including detoxification enzymes and protective enzymes, to eliminate reactive oxygen species and resist UV stress. Additionally, insects can protect their bodies through DNA repair when intense UV light causes DNA damage[71], as shown in Fig. 1.
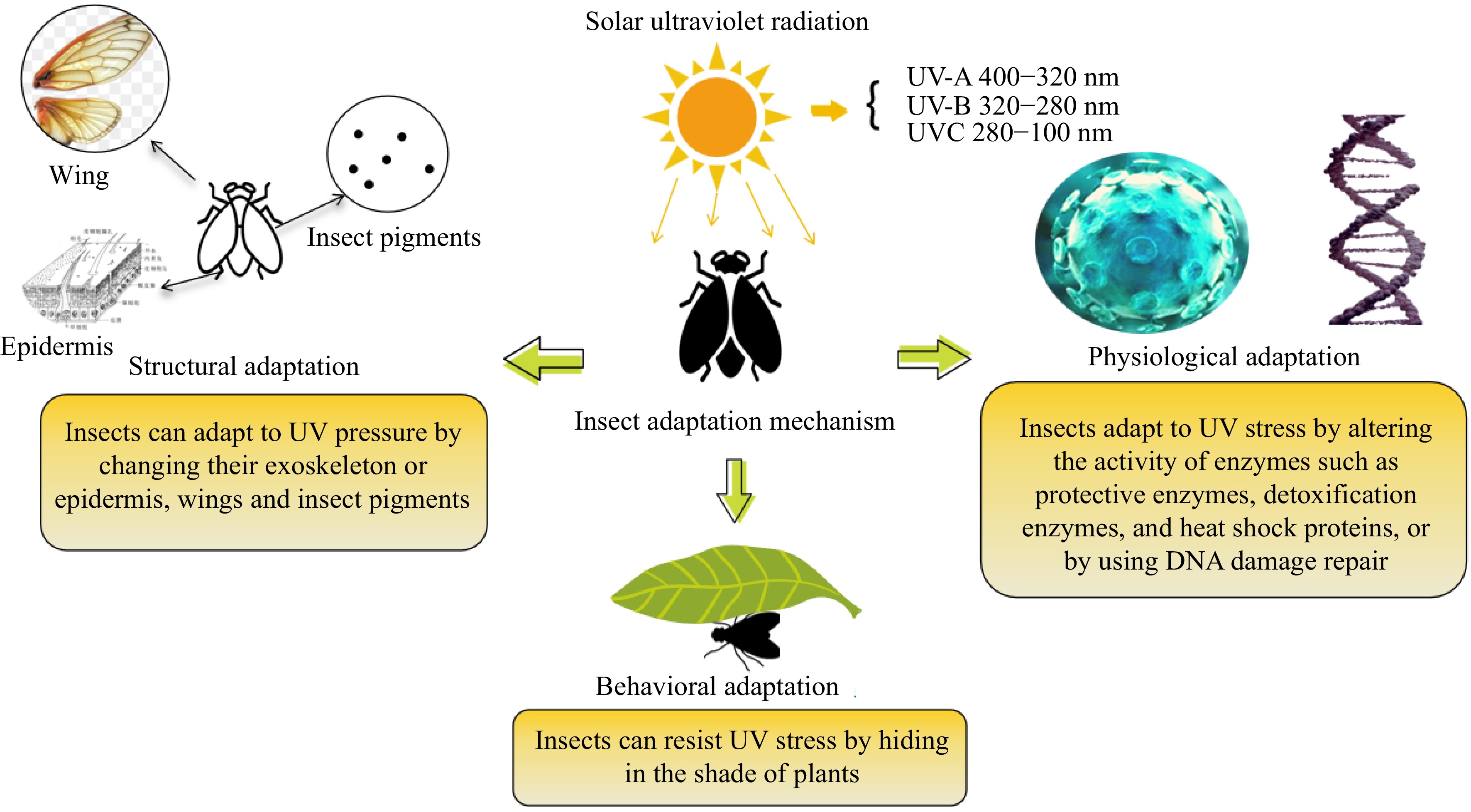
Figure 1.
Adaptation of insects to ultraviolet radiation. Note: Figure modified from The biological effects of ultraviolet light on insects according to Wang et al.[69].
Harman first proposed the free radical theory in 1956. This theory posits that free radicals can destroy biological macromolecules such as proteins and lipids in the body, which results in the loss of organelle function[70]. Free radicals are also considered to be the culprit leading to biological aging and death. UV stress can induce excessive production of ROS in insects[72]. Insects, like other eukaryotes, have developed a set of antioxidant systems to combat the toxicity of ROS. When insects are under UV stress, they mainly rely on the antioxidant system in their body to remove ROS and prevent oxidative damage caused by UV. The concept of the antioxidant enzyme system was proposed by Fridovich in 1997 as a result of the incomplete reduction in oxygen and represents the main self-protection mechanism[73].
Antioxidant systems can be divided into two different types: enzyme-promoted and nonenzyme-promoted systems[74]. The nonenzymatic antioxidant system in insects is mainly composed of antioxidants such as glutathione, vitamin C, vitamin E, and carotenoids; it not only can reduce ROS but also can serve as a coenzyme-assisted enzymatic antioxidant system in insects[75]. An enzymatic oxidation system composed of protective enzyme systems such as SOD, POD and CAT exists in insects, and this system can quickly remove ROS produced in the body and reduce oxidative damage to insects[76]. The three protective enzymes cooperate and coordinate with one another, as shown in Fig. 2. They form the core of the antioxidant system in insects and jointly maintain the homeostasis of physiology and biochemistry in insects.
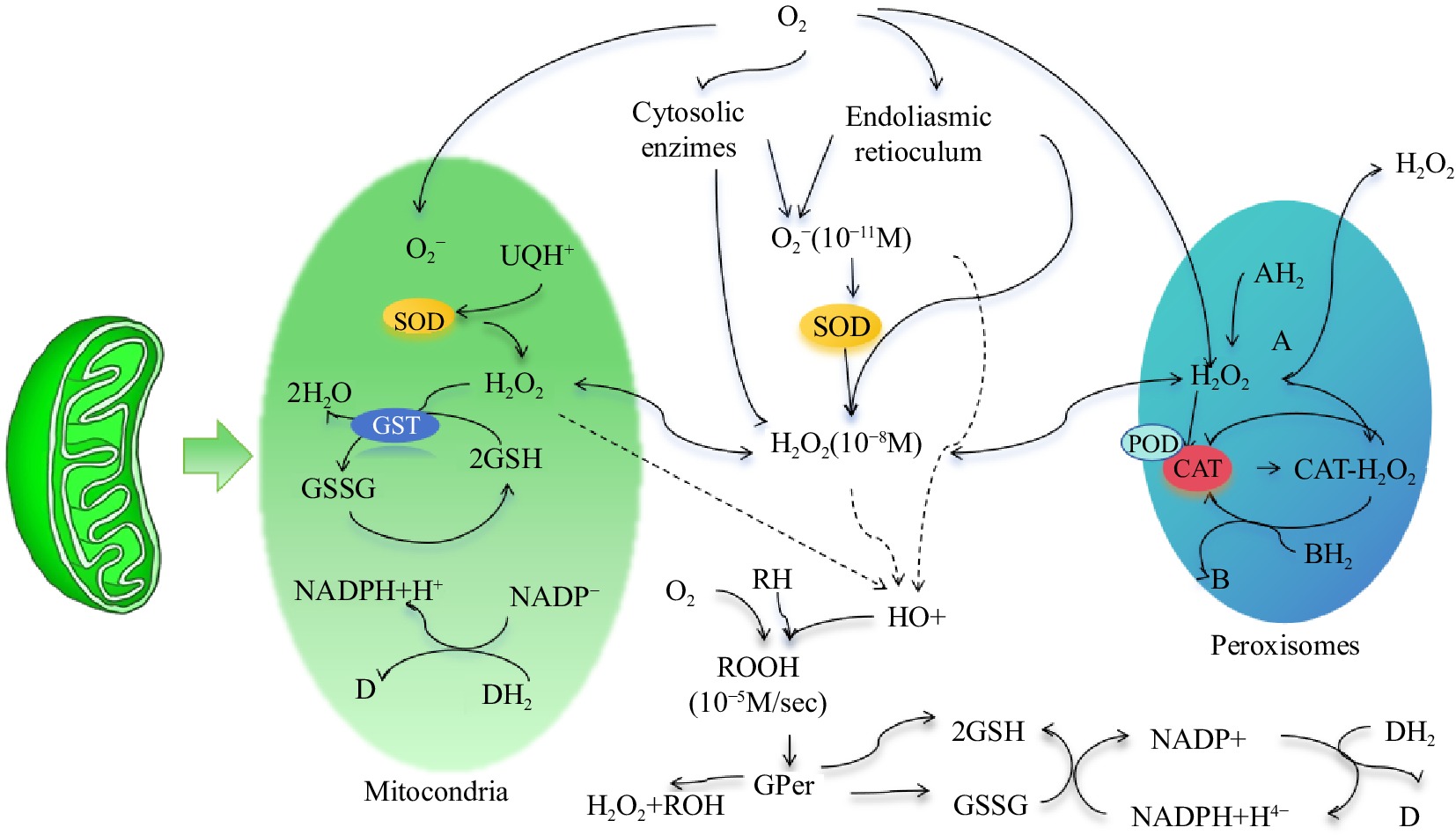
Figure 2.
Distribution and interaction of SOD, POD, and CAT protective enzymes in cells. Note: Figure Modified metabolic pathways of hydrogen peroxide metabolism in cells according to Valdez et al.[77].
Studies show that low levels of ROS are not harmful to cells, are conducive to maintaining the normal operation of biological organisms, and are an indispensable part of cell signaling and inducing host defense genes[7]. However, when ROS levels may rise sharply under stress far beyond the capacity of the ROS scavenging system, excessive free radicals in organisms may lead to disruption of the balance of ROS[78]. A large amount of accumulated ROS accelerated the process of free radical chain reaction in peroxidation of cell membranes, which produced a large number of unstable lipid peroxidation intermediates and caused great damage to the structure and function of cell membranes[79]. In addition, the accumulation of ROS to a certain extent can damage DNA and lead to gene mutation[80]. UV leads to the photooxidation of endogenous photosensitivities. Although the absorption of UV radiation abovementioned 320 nm by nucleic acid bases is weak, UV-B radiation in the 280–320 nm range can damage DNA by forming cyclobutane pyrimidine dimers and 6−4 photoproducts on adjacent thymidine residues. ROS is produced efficiently in aqueous media[31]. The antioxidant enzyme defense system in insects can react with free radicals produced by lipid peroxides, which stops the chain reaction[81].
Some domestic scholars have studied the mechanism of insect enzymes responding to UV stress and found that SOD is one of the most important enzyme systems in insect response to UV stress[82]. The operation of SOD is inseparable from the assistance of CAT and POD, which jointly maintains the antioxidant system of insects. All three protective enzymes are necessary. When SOD reduces a large amount of superoxide anions in insects to H2O2, excessive H2O2 will also cause damage to the body. When H2O2 reaches a high concentration, CAT is responsible for its decomposition into water and oxygen that is harmless to the body, while POD is responsible for the decomposition when H2O2 drops to a low concentration level. The abovementioned process was verified through experimentation. At the early stage of stress, POD activity was significantly higher than that of the control, while CAT activity was significantly lower than that of the control. At this time, POD competitiveness was greater than that of CAT, and the rapid reduction in H2O2 concentration led to the inhibition of CAT activity. However, POD activity decreased while CAT activity increased significantly after long-term UV irradiation with the rise in UV irradiation time, which suggests that CAT played a more important role than POD in the process of H2O2 removal when insects were exposed to UV irradiation for a long time[24].
Studies have shown that the operation of the antioxidant enzyme system under the selective pressure of adversity is a complex process of multienzyme cooperation, and a single enzyme cannot offer a complete protective function. The enzymes that protect the enzyme system are closely related and complement each other, and the change in the activity of one enzyme will lead to the change in the activity of other enzymes. The results of the study on the changes in insects under different durations of UV stress showed that the response mechanism of insects to UV radiation was not fixed but changed with the variation in irradiation time, and different response modes would be different under different durations of UV stress. Su obtained 157 differentially expressed genes after irradiating Ostrinia furnacalis for 1 h with UV-A, and 637 differentially expressed genes after 2 h with continuous irradiation[83]. Similar results were obtained by Sang using UV-B irradiation of different duration. This finding suggests that the genes involved in the response to UV radiation in insects increase with the duration of UV stress[84]. In addition, the multiples of differentially expressed genes change with the variation in UV radiation. Lopez-Martinez et al. found sustained and strong expression of SOD-encoding mRNA in insects living in Antarctica with high intensity UV radiation under UV stress conditions, which means that insects living in UV stress environments for a long time have evolved a mechanism to prevent oxidative stress by producing large amounts of antioxidant instead of responding to stress by turning on the transcription of the SOD gene[78]. Given that insects had already maximized the expression of the gene under long-term UV stress, none of the environmental stressors examined led to further upregulation of the gene.
Insect response to UV stress is not only dependent on antioxidant action but also a complex system of multiple resistance pathways and multigene synergies. In addition to the detoxicating and protective enzymes responding to UV stress in insects, some enzymes such as heat shock proteins (Hsps) and mitogen-activated protein kinases (MAPKs) are also involved in insect response to UV stress[85]. Yang found that four MAPK signaling pathway genes and eight MpHsp70s genes were involved in the functional expression of M. persicae in response to UV-B stress[86]. The SfHsp90 gene of Spodoptera frugiperda plays an important role in the molecular mechanism of response to environmental stress[87]. Zhao et al. found six types of genes involved in UV stress in M. persicae[44]. In addition, coenzyme NADH, oxidoreductase, and epidermal protein showed significant differences after UV treatment. In addition to the changes in related gene expression and protective and detoxification enzyme activities, many complex physiological and biochemical changes occur, such as tyrosine metabolism, glycolysis, and gluconeogenesis in insect response to UV stress[18].
At present, most studies on insect detoxification enzymes home and abroad focus on drug resistance, while relatively few works have been conducted on the changes in detoxification enzymes under UV stress conditions. The mechanism of insect detoxification enzymes responding to UV stress is still unclear and needs further research.
-
Relevant studies at home and abroad have shown that the ROS in insects increased after short-term UV stress, and insects can adapt to the stress by improving the enzyme activity to clear the ROS. The activity of SOD, CAT, and POD, the total antioxidant capacity of enzymatic antioxidant system, and gene expression was significantly increased in insects. After UV irradiation over a long period, the activity of SOD, CAT, and POD gradually decreased, and the high concentration of enzyme substrate would have a negative feedback regulation effect on the protective enzymes. Ultimately, the enzyme activity decreased. At present, relatively few studies focus on the changes in detoxification enzymes under UV stress. Existing works have shown that short-term UV stress can inhibit the activities of AChE, CarE, and GST in insects to a certain extent, and the activities of AChE, CarE, and GST will increase to a certain extent with the rise in UV irradiation time. However, the detoxification enzyme activity was finally inhibited after long-term UV stress. The activity of GST was significantly higher than that of AChE and CarE after UV stress. Therefore, GST played a greater role in insect detoxification enzyme response to UV stress. Studies on the changes in insect CYP450 under UV stress mainly focus on the effects on CYP450 gene expression. Works have shown that UV had different effects on different CYP450 genes in insects. The changes in detoxification and protective enzyme systems in insects under UV stress are also related to insect species and sex.
The mechanism of insect response to UV stress is a complex system of multiple resistance pathways and multigene synergies. It has different patterns in different time periods and involves various complex biological processes such as the gene expression, protein translation, and physiological changes. The detoxification and protective enzyme systems in insects complement each other and jointly maintain the stable operation of the antioxidant system. Changes in the activity of one enzyme in the body may lead to alterations in the activity of other enzymes to a certain extent. Therefore, the changes in various enzyme activities and the content of antioxidant substances need to be considered simultaneously to obtain a correct conclusion when exploring the adaptation of insects to stress.
Protective and detoxification enzymes also play an important role in insect adaptation to other adverse conditions such as high CO2 and high temperature. When the concentration of CO2 in the environment increased, the activities of the protective enzymes POD and CAT and the detoxification enzymes CarE and AChE in Frankliniella occidentalis adults increased significantly[88]. The AChE and GST activities of Trabala vishnou gigantina showed an increasing trend under high CO2 concentration[89]. High temperature significantly inhibited the activity of three detoxification enzymes, namely, AChE, CarE, and GST, and the contents of CYP450 in insects[90]. Li found that the activities of the three protective enzymes SOD, CAT, and POD in N. barkeri were upregulated at 40 °C, and all showed a trend of first increasing, then decreasing, and finally increasing[91]. Among the three protective enzymes, SOD was the most sensitive to temperature stress and rose rapidly and reached the maximum value in a short period of time, followed by CAT and POD that reached their peaks[92]. Wang determined that the specific activity of detoxification enzymes in insects increased after high-temperature stress treatment, and appropriate high temperature could increase or decrease insect resistance to pesticides. Similar prolonged exposure to UV stress is also likely to interfere with pesticide detoxification mechanisms in insects, which makes them change their sensitivity to pesticides[93].
In summary, protecting and detoxifying the enzyme system in the defense measures of insect adaptation to stress conditions is important. At present, no systematic study has been conducted on the mechanism of the insect enzyme system responding to UV light stress. Follow-up studies can be devoted to revealing the protective mechanism of the insect enzyme system and other substances. Understanding the effects of UV stress on the protective and detoxification enzyme systems in insects can provide new ideas for pest management, such as using UV stress to develop appropriate physical control strategies, to provide theoretical support for green prevention and control technology.
-
The authors confirm contribution to the paper as follows: Dong WB prepared and edited the manuscript with assistance from Li F and Wu S; Hou D, Hou Q and Jin H, with the support of Li F, were responsible for the collection of relevant data. All authors read and approved the final version of manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
The financial support for this study was provided by Hainan Major Science and Technology Project (ZDKJ2021007),Project of Sanya Yazhou Bay Science and Technology City (SCKJ-JYRC-2023-15), Hainan Province Key Research and Development Project (ZDYF2021XDNY190) and National Key Research and Development Program of China (2022YFD1401200 and 2022YFD1400900).
-
The authors declare that they have no conflict of interest.
-
Received 21 September 2023; Accepted 11 February 2024; Published online 15 March 2024
-
# Authors contributed equally: Fen Li, Shaoying Wu
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Dong W, Hou D, Hou Q, Jin H, Li F, et al. 2024. Effects of ultraviolet light stress on protective and detoxification enzymes in insects. Tropical Plants 3: e007 doi: 10.48130/tp-0024-0008
Effects of ultraviolet light stress on protective and detoxification enzymes in insects
- Received: 21 September 2023
- Revised: 23 November 2023
- Accepted: 11 February 2024
- Published online: 15 March 2024
Abstract: The intensification of environmental pollution and the destruction of the ozone layer has increased the amount of ultraviolet (UV) radiation reaching the surface daily, which resulted in great selective pressure for plants and insects in the biosphere. UV light stress can seriously affect the growth and development of insects, such as growth retardation, fecundity weakening, and life span shortening. Protective and detoxification enzymes, which are two important adaptive enzyme systems in insects, play a dominant role in regulating insect response to UV stress. On the basis of domestic and foreign studies on the changes in detoxification and protective enzymes under UV light stress, the effects of UV stress on the activities of protective and detoxification enzymes in insects and the mechanism of insect response to UV light stress are introduced in this study. Thus, this work provides reference suggestions for the application of UV light in comprehensive pest control and promotes the development of green prevention and control technology.












