-
Nuclear power plants play a critical role, not only in electricity supply, but also in achieving peak carbon emissions and carbon neutrality. The sustainable supply of nuclear fuel is crucial for the healthy development of nuclear energy utilization. Uranium-235 is one of the most important radionuclides for the production of nuclear fuel. However, terrestrial uranium resources can only sustain current demand for approximately 70 years. There are about 4.5 billion tons of uranium in the ocean, which is enough to guarantee the peaceful utilization of nuclear energy for thousands of years[1]. However, the efficient extraction of uranium from seawater remains a worldwide challenge because of the extremely low uranium concentration, and presence of other metal ions and microorganisms[2]. Porous carbon-based materials are considered one of the most promising candidates for uranium preconcentration from seawater because of their porous structures, specific functional groups, and active sites for the highly selective binding of uranyl ions through chemical complexation, reduction, and precipitation strategies[3].
A recent study by Guo et al.[4] introduces an innovative strategy for enhancing uranium recovery from seawater by focusing on structural geometry and chemical surface functionality. By deliberately controlling the stacking mode of sulfonic covalent organic frameworks (S-COFs), the authors successfully constructed a uranyl-recognition pocket that precisely matches the planar coordination preference of uranyl ions through four coordination sites in the presence of other competing ions in natural seawater. The AB stacking mode provides a pocket for precise recognition of uranyl ions, with the inner pore size of 0.9 nm and a pocket arrangement for –SO3H unites to selectively bind uranyl ions by chemical coordination with high sorption kinetics (Fig. 1a). In the AB stacking configuration, sulfonic functional groups are arranged in a unique 3D orientation, producing a confined cavity that provides a planar 4-fold coordination environment. This AB stacking structural feature leads to exceptionally strong binding affinity, ultrafast adsorption kinetics, and record-high distribution coefficients—approximately 1,000-fold higher than those of the AA stacking mode—suggesting the significant contribution of AB stacking mode. Importantly, the AB-stacked S-COFs also demonstrate remarkable selectivity against vanadyl ions and other competing ions, overcoming one of the major barriers in seawater uranium extraction. The authors obtained ultra-high uranyl affinity with a Kd value of 1.0 × 1010 mL/g and a U(VI)/V(V) selectivity of 1,000, the highest values among currently reported materials. More importantly, a record extraction capacity of 31.5 mg/g U(VI) from natural seawater was achieved in just one day, much higher than any todays' reported values (Fig. 1b). XAFS characterization indicates 2-fold coordination in the orbital plane and 4-fold coordination in the equatorial plane, similar to that of UO2(NO3)2·6H2O (Fig. 1c). Further DFT calculation studies reveal that uranyl is fixed by a precise uranyl-identification pocket, forming 4-fold coordination with U-O bond distances of 2.42–2.58 Å, in good agreement with XAFS analysis. The high sorption energy (–9.83 eV) of uranyl on S-COF-AB indicates the strong binding of uranyl ions, much higher than that for other metal ions (Fig. 1d). Comparing to S-COF-AB, S-COF-AA uses only one –SO3H site to bind uranyl ions, with lower sorption energy (–6.60 eV), suggesting weaker affinity of S-COF-AA to uranyl ions. The sorption energies of other metal ions by S-COF-AB and S-COF-AA also confirm the higher selectivity of S-COF-AB to uranyl ions. The uranyl-specific pocket not only ensures efficient uranyl capture under realistic seawater conditions, but also maintains stability and antifouling capability, making it highly promising for long-term real applications. The integration of structural precision with functional performance highlights the pivotal role of supramolecular geometry in adsorption science. Overall, this study pioneers the concept of 'stacking-mode engineering' in COFs, establishing a new design paradigm for constructing ion-specific recognition motifs.
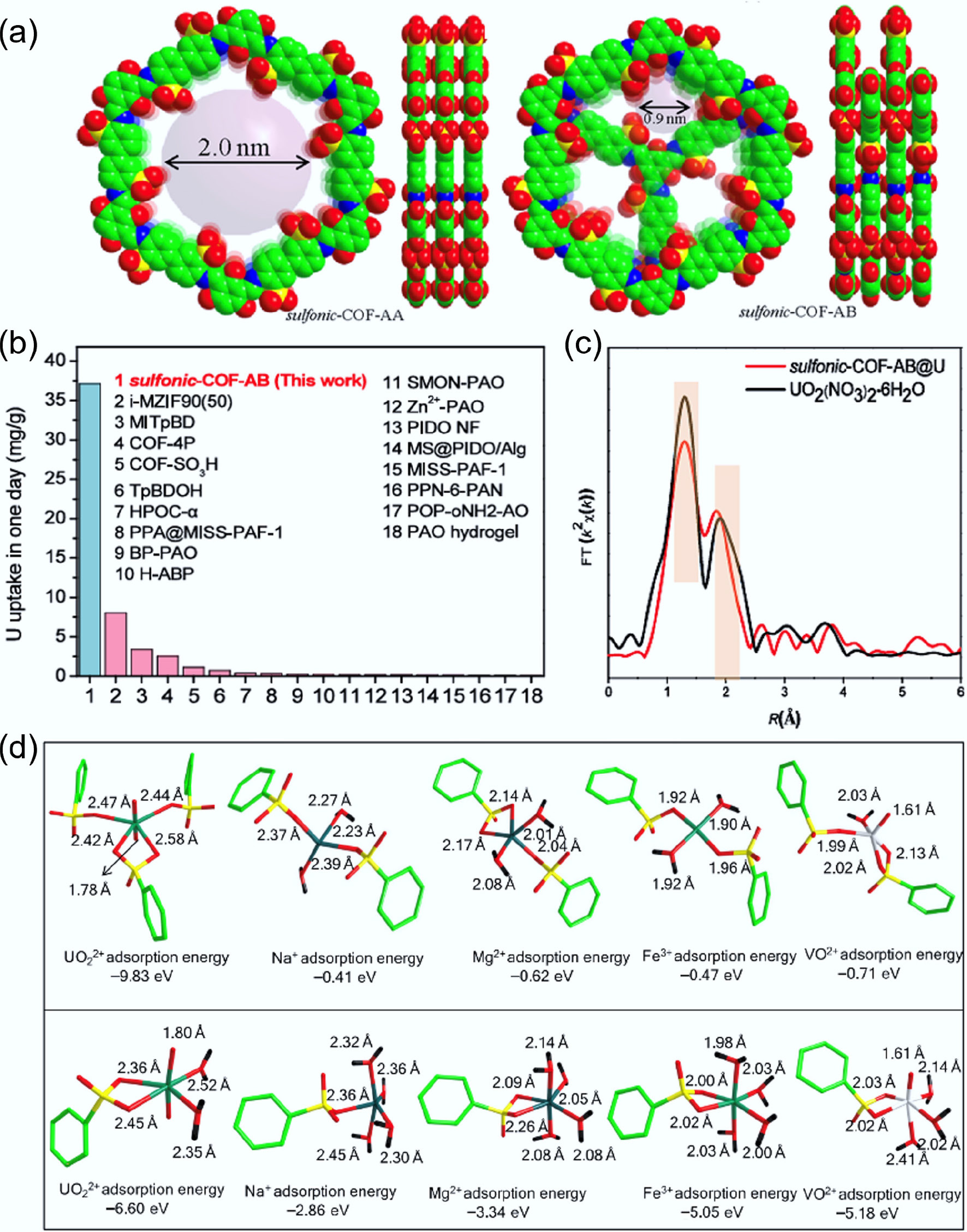
Figure 1.
(a) Top and side views of S-COFs with AA and AB stacking modes. (b) Comparison of uranyl extraction by S-COFs-AB from seawater in one day with other material. (c) EXAFS analysis of S-COF-AB@U and UO2(NO3)2·6H2O. (d) Sorption structures of UO22+, Na+, Mg2+, Fe3+, and VO2+ in S-COF-AB (up) and S-COF-AA (down) with corresponding sorption energies, respectively[4].
For real applications, the high sorption capacity of nanomaterials is related not only to the tunable porous structures and functional special groups, but also to the stability and reusability. In seawater uranium extraction, the fiber materials modified with amidoxime groups (for high selective binding of uranyl ions), active sites (to improve sorption ability), light absorption property (for antibacterial activity and photocatalytic reduction ability), electroconductivity (for electrochemical extraction), porous structures (for fast transfer of uranyl ions), low cost at industrial level synthesis (for real application on a large scale), high stability (against the possible loss and reduce potential pollution in the ocean), reusability (to reduce the cost) etc., are promising materials in ocean applications. At the laboratory level, high extraction ability can be easily achieved; however, the way for ocean uranium extraction with low economic level, and high ability is still a huge challenge for nuclear energy utilization. We believe that uranium extraction from the ocean will open a new way for nuclear fuel preparation with the development of technology and science.
HTML
-
The authors confirm their contributions to the paper as follows: Xishi Tai drafted the initial manuscript; Zhenli Sun reviewed and revised the manuscript. Both authors contributed to the final version and approved it for publication.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
-
None to declare.
-
The authors declare that they have no conflict of interest.
-
Full list of author information is available at the end of the article.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Tai X, Sun Z. 2025. Extra-high extraction of uranium from seawater by covalent organic frameworks through structure geometry and functional active site modification. Sustainable Carbon Materials 1: e006 doi: 10.48130/scm-0025-0007 |












