-
Climate change is diminishing crop yield and escalating the cost of agricultural products, and an additional 77 million people are at risk of food insecurity by 2050[1]. Significant increase in global temperature and the emergence of additional abiotic stressors adversely affect crop yield. In this context, eco-friendly technologies and sustainable farming practices offer a promising solution, enhancing yields under more challenging conditions and improving resource utilization[2]. The goal is to maintain plant health by sequestering carbon, preserving and enhancing soil organic matter and mineral content, and promoting healthy crop yields while reducing detrimental inputs[3,4]. While certain plants have developed the resilience to tolerate environmental fluctuations and thrive in harsh conditions, most crops will likely experience reduced productivity as environmental stresses surpass their ability to adapt. Distinctive bacterial genera are essential constituents of the soil ecosystem[5], playing a key role in various biotic activities that enhance turnover and support crop production[4,6]. These bacteria enhance plant growth by increasing nutrient availability in soils, synthesizing plant growth regulators, protecting plants from phytopathogens through regulating plant defence or directly inhibiting them, and reshaping soil composition. Additionally, they contribute to bio-remediating the contaminated soils by sequestering toxic metal species[7]. Root-related microorganisms, including endophytes, interact closely with one another and play a crucial role in regulating significant physiological procedures[8], particularly nutrient acquisition and plant resilience towards abiotic stresses[7,9]. Plants inhabited by plant growth promoting rhizobacteria (PGPR) typically exhibit increased root hair development, hence enhancing the absorption of minerals and micronutrients from the soil[10].
The study on rhizobacteria which colonize plant roots and regulate plant health, has significantly advanced during the past century. Understanding plant-microbe interactions began in the late 19th and early 20th centuries with soil microbiology research. Beijerinck discovered nitrogen-fixing Rhizobium species in legume root nodules, revealing soil bacteria's symbiotic potential to boost plant growth[11]. Kloepper & Schroth coined the name PGPR after discovering free-living bacteria that promoted plant development in the 1960s and 1970s[12]. Research has expanded our understanding of rhizobacteria roles including both beneficial and harmful species. Nitrogen fixation, phosphate solubilization, hormone generation, and pathogen defense by beneficial rhizobacteria like Pseudomonas, Bacillus, Azospirillum, and Rhizobium help plants flourish[13]. Ralstonia solanacearum and Agrobacterium tumefaciens, on the other hand, cause bacterial wilt and crown gall, respectively. While PGPR are vital for sustainable agriculture, their presence and effectiveness are significantly affected by environmental conditions[14]. Soil pH, temperature, moisture levels, and nutrient availability also impact the microbial community makeup and decide whether beneficial or detrimental microorganisms will prevail. Under appropriate conditions, PGPR can outcompete pathogenic bacteria and form beneficial associations with plants[15]. However, environmental stressors such as drought, salinity, and pollution may alter microbial populations, sometimes favoring pathogenic bacteria or diminishing PGPR efficiency[16]. Optimizing plant-microbe interactions in agricultural and natural settings requires a thorough understanding of rhizobacteria, including its pros and cons. Therefore, there is a need to improve PGPR performance and reduce bacterial impacts by comprehensively reviewing rhizobacteria history and functions.
PGPR treatment has significantly improved the cultivation of various plants[17], and established foundational resilience to a range of abiotic stresses in plants, often through modulation of plant physiology[18]. Leguminous plants and microbes have a significant deal of potential to improve the quality and richness of soil[19] through symbiotic co-development and bio-mineralization[20]. It is necessary to investigate the potential of these rhizobacteria in soil to mitigate plant pathogens under fluctuating climatic conditions and to enhance plant growth through various direct and indirect mechanisms, considering the increasing impact of climate change and the introduction of new pathogens[21,22]. An integrated method could be employed for transplanted vegetables to produce healthy transplants that are tolerant to nematodes and various infections for a minimum of several weeks post-transplantation to the field[23]. Selected strains of putative PGPR activate plant-induced systemic resistance (ISR) effectively against multiple plant diseases. ISR is a plant-mediated mechanism akin to traditional pathogen-induced resistance, wherein uninfected regions of previously infected plants gradually acquire enhanced resistance to subsequent diseases[24].
PGPR is now essential in sustainable agriculture since it enhances plant development and improves soil health through multiple mechanisms. However, the changing climate is posing substantial threats to these helpful microbes. Climate change dramatically alters the environmental conditions that bacterial communities inhabit. These alterations impact bacterial communities diversity, abundance, and functionality[25]. Increasing temperature, altered rainfall patterns, and increasing carbon dioxide levels contribute to changes in bacterial population structure. Consequently, some species might be lost while others proliferate, disrupting ecological balances. Climate-induced changes in bacterial community diversity could result in a shift in mutualistic interactions, potentially reducing plant fitness. Meanwhile, plants can influence the composition and structure of associated bacterial communities through root exudation, creating a dynamic feedback loop. During eco-evolutionary events, bacteria might have undergone several selection pressures where beneficial interactions with host plants shape the hemostasis in the microbiome (Fig. 1). Hereby, we aim to examine the influence of climate change on PGPR and emphasize its effects on their diversity, functionality, and plant-microbe interaction. By understanding these effects, we can develop strategies to reduce the harmful impact of climate change and improve the resilience of agricultural systems. Considering the significant role of PGPR in stress and plant growth development by combating plant diseases and enhancing agriculture productivity, it is important to explore and highlight their significant role in biotic and abiotic stresses and overall plant growth development.
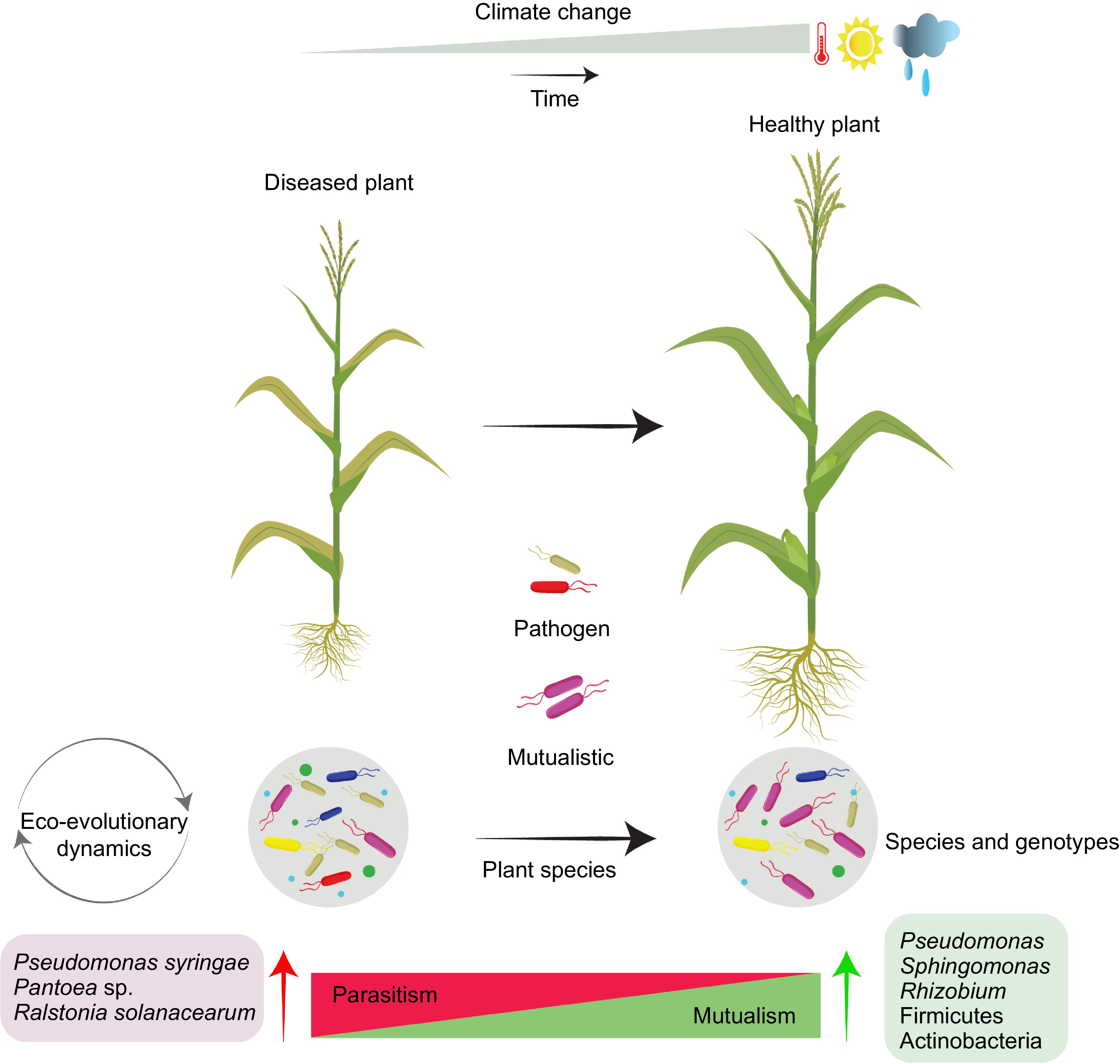
Figure 1.
Evolution of plant-PGPR interactions under climate change.
Climate change can impact eco-evolutionary dynamics of plant growth promoting bacterial communities. Plant-associated PGPRs have been evolving with host plants to better adapt and perform beneficial activities contributing to improved health status. The eco-evolutionary selection of species and genotypes can modulate plant-microbe interactions and by selecting mutualistic bacteria along parasitism-mutualism continuum. -
Phytopathogenic microorganisms pose a significant and constant threat to agribusiness and ecosystem stability, subvert the soil biology, disturb plant conditions, degrade soil productivity, and thus may result in harmful consequences for human wellbeing alongside contaminated groundwater[26]. PGPR is a promising and ecologically well-suited alternative to improve plant development and continual fertility of the soil. The microbes colonizing the rhizosphere include parasites, algae, bacteria, protozoa, and actinomycetes. However, bacteria are the most plentiful and well-studied microorganisms in the rhizosphere[27]. The improvement of plant development using these microbial interventions has been widely demonstrated (Table 1). The utilization of PGPR can accomplish sustainable agriculture development as a biofertilizer inoculant[28]. Commonly, bacteria endorse plant growth in three unique ways: encouraging the uptake of soil nutrients, producing plant growth promoting hormones, and reducing or preventing plants from diseases[29]. The in-depth complex mechanisms of plant development by PGPR are still fragmentary. But the potential explanation includes biological nitrogen fixation, solubilization of minerals phosphate, potassium, and many others, sequestering iron, phytohormone production like cytokinin, auxin, gibberellins, abscisic acid (ABA), reduced levels of ethylene in roots for plant development by the production of ACC- deaminase, antibiotics, and resistance to environmental stresses[30].
Various stresses arise from soil, impacting plant development and playing a significant role in sustainable agriculture production. These stresses can be characterized as biotic and abiotic. Abiotic stress is the essential driver of yield loss worldwide by over 30%[31]. According to research, climate change has a substantial impact on the composition, diversity, and function of plant-associated rhizobacteria, influencing plant health and productivity. Temperature, precipitation, and CO₂ levels can alter soil microbial populations, causing variations in plant-microbe interactions[32,33]. This can limit plant growth promoting benefits while increasing plant vulnerability to diseases. As climate change continues to affect ecosystems, understanding the effects on plant-microbe interactions becomes increasingly important[34,35]. Here, we illustrated the importance of adaptation techniques, such as microbial inoculants and biostimulants, for maintaining plant productivity in the face of changing environmental conditions. The most prevalent factors in plant growth and productivity are abiotic stresses caused by salinity, drought, and high temperatures[36]. The application of PGPR in plant abiotic stress management has been extensively considered through bacterial strains, for example, Pseudomonas fluorescens and P. putida that reduced the lethal impact of cadmium contamination on barley plants through bioremediation of cadmium particles from soil[37,38]. In addition, these bacteria have been shown to improve leaf water status, especially under salinity and other abiotic stress conditions[39]. Various pathogens incite biotic stresses, for example, fungi and protists, resulting in a critical decrease in crop yield[40,41]. Biotic stress severely affects plants, including population dynamics, biological system supplement cycling, natural habitat ecology, co-evolution, and agriculture plant health[42]. Plants inoculated overnight with PGPR cultures depicted tremendous protection from biotic stresses[43,44].
Table 1. PGPR-host plant-stress interactions.
PGPR species Host plant Environmental stress Mechanisms of action Ref. Pseudomonas fluorescens Rice (Oryza sativa) Drought Induced root elongation, enhanced water retention, increased ABA production [45] Bacillus subtilis Maize (Zea mays) Pathogen-induced stress Production of antifungal compounds, induced systemic resistance [46] Azospirillum brasilense Wheat (Triticum aestivum) Salinity Improved ion homeostasis, enhanced antioxidant enzyme activity [47] Rhizobium sp. Alfalfa (Medicago sativa) Soil degradation, nutrient deficiency Increased nitrogen fixation, enhanced rhizosphere microbial diversity [48] Priestia aryabhattai Alfalfa (Medicago sativa) Drought Increased rhizosphere carbon sequestration, improved soil health [48] Pseudomonas putida Barley (Hordeum vulgare) Drought Production of exopolysaccharides, root microbiome modulation [49] Bacillus amyloliquefaciens Lettuce (Lactuca sativa) Salinity Phosphate solubilization, zinc uptake enhancement [50] Paenibacillus polymyxa Sorghum (Sorghum bicolor) Heat stress Modulation of stress-responsive genes, phytohormone regulation [51] Bacillus velezensis Tomato
(Solanum lycopersicum)Heavy metal stress Bioremediation, metal ion sequestration [52] Serratia marcescens Barley (Hordeum vulgare) Drought ACC deaminase production, reduced ethylene stress [53] Acinetobacter calcoaceticus Sunflower
(Helianthus annuus)Heavy metal toxicity Cadmium and lead immobilization, production of metal-chelating siderophores [54] Enterobacter cloacae Tomato
(Solanum lycopersicum)Drought Synthesis of osmo-protectants (proline, trehalose), increased antioxidant activity [55] Klebsiella variicola Wheat and maize Salinity Regulation of Na+/K+ balance, secretion of growth hormones [56] Bradyrhizobium japonicum Soybean (Glycine max) Heat stress Increased nitrogen fixation, stabilization of chlorophyll pigments [57] Climate changes the composition and functionality of rhizobacterial communities, which can improve or hamper plant-microbe interactions. Environmental stressors like elevated temperatures, precipitation patterns, and CO₂ levels can alter microbial community structures, favoring stress-tolerant taxa and decreasing diversity[58]. Certain bacterial phyla respond differently to environmental changes. Increased CO₂ levels and moderate warming generally lead to increased Proteobacteria abundance, likely due to their versatility in nitrogen fixation and plant growth stimulation[59]. Members belonging to Pseudomonas and Rhizobium improve nutrient absorption and protect plants from diseases, suggesting that moderate temperature shifts may temporarily boost plant-microbe interactions. However, prolonged or acute environmental stress can alter these interactions, causing unanticipated plant health and soil stability effects. Under drought and nutrient-poor conditions, Verrucomicrobia, a phylum responsible for soil carbon cycling and nutrient turnover, declines, threatening soil microbial network stability and ecosystem resilience[60,61]. Bacteroidetes, which decompose organic matter, also decrease in abundance during significant temperature swings, which may lower soil fertility and nutrient availability[62]. In contrast, Actinobacteria and Firmicutes, which include stress-tolerant genera like Streptomyces and Bacillus, often increase in abundance in arid and degraded soils due to their ability to produce secondary metabolites and antibiotics that help plants resist pathogens and abiotic stress[63]. In progressive soil deterioration, these taxa may compensate for plant defensive mechanisms. Multiple studies have found that harsh climate conditions lower microbial diversity, which reduces ecosystem resilience and plant development[64]. Disrupting mutualistic rhizobacterial relationships may decrease plant stress responses, making them more susceptible to infections and lowering plant productivity[65]. However, some PGPR strains (Pseudomonas and Rhizobium) have been shown to promote root colonization, suggesting that these rhizobacteria may ameliorate climate-induced stress on plants[66]. These modifications must be understood to develop climate-change-resistant soil, microbial health, and plant production methods. Biostimulants and microbial inoculants are being used in microbial biotechnology to prevent these alterations and improve plant-microbe interactions in changing environments[67]. The molecular basis of these microbial adaptations and their long-term effects on plant health and agricultural sustainability should be studied.
-
In the era of climate-related catastrophic events, exploring new mechanisms of bacterial plant growth development that can provide important nutrients to plants, such as fixed nitrogen, phosphorus, potassium, and iron, is crucial[68]. Numerous cultivating soils do not contain an adequate quantity of at least one of these nutrients, so plant growth is halted[69]. Farmers have increasingly relied on chemical sources of these nutrients[70] to obtain higher plant yields. Beneficial and productive natural methods for providing nutrients to plants could be utilized to substitute for at least a part of the chemical nutrient fertilizers. The use of microbial technology is diminishing the requirement for agrochemicals (composts and pesticides) to improve soil fertility through mechanisms such as siderophores, hydrolytic catalysts, antibiotics production, and HCN[71]. The positive effects of rhizobacteria on plants involve complex interactions that can enhance plant growth and mitigate stress. These interactions can be broadly categorized into direct and indirect mechanisms of plant growth promotion. Direct activities fostering plant growth include producing phytohormones, solubilizing nutrients, and biological nitrogen fixation (Fig. 2). In contrast, factors such as systemic resistance against pathogens and niche diversification represent indirect mechanisms.
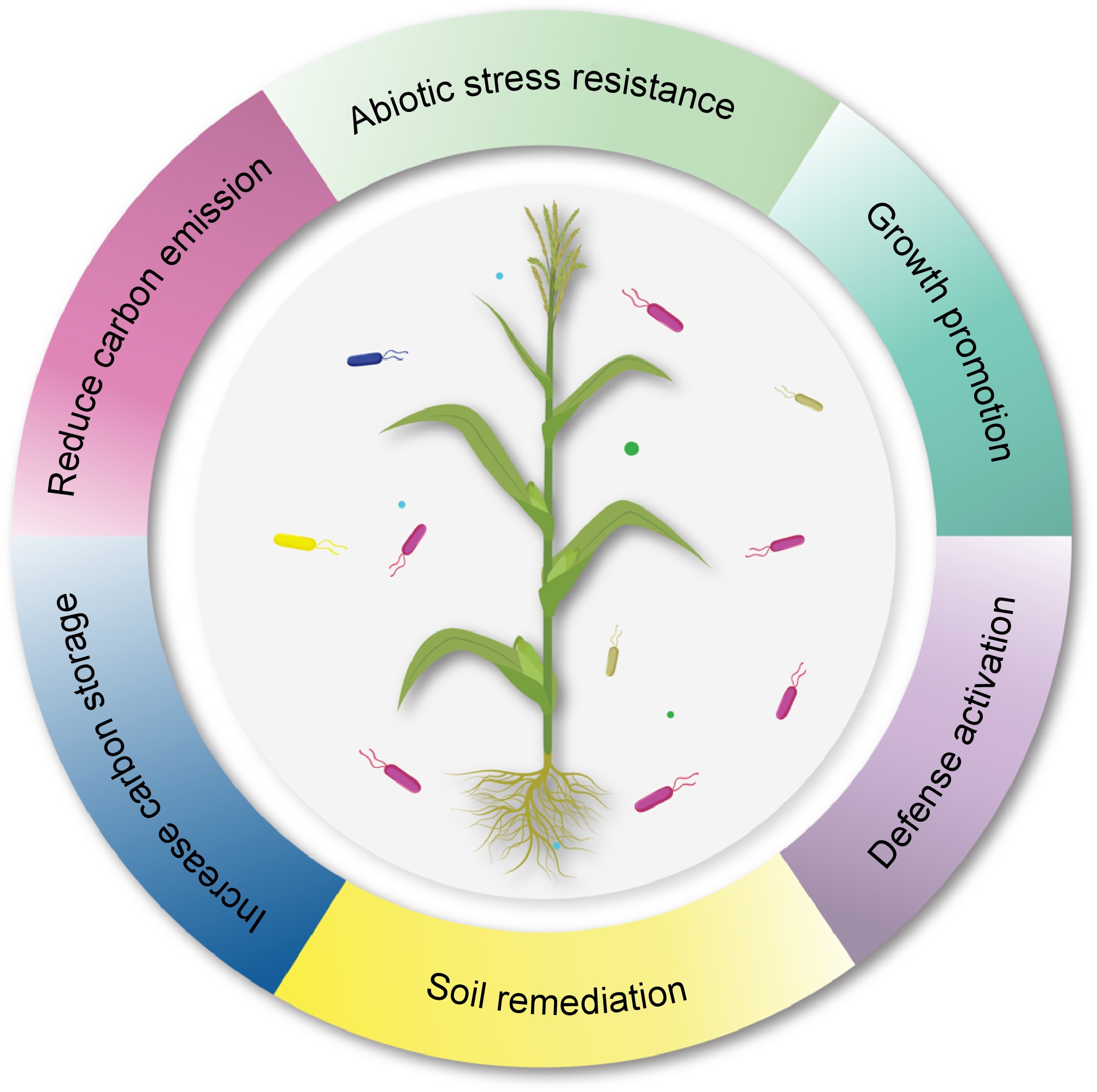
Figure 2.
Ecological and plant benefiting services of plant growth promoting bacteria.
PGPR can significantly alleviate the impact of climate change through improving carbon storage in soil or carbon sequestration and by reducing the carbon emission. Further, PGPR can also enhance the plant resistance towards climate induced abiotic and biotic stresses through defense activation and improving plant vigor. They also prevent plant from negative effects of toxic materials by soil bioremediation.The soil and plant-associated microbial partners are foreseen to play key roles in maintaining sustainability via the intricate interplay of mutualism and cooperation with their host plants. Among them, rhizobacteria are the free-living bacteria in the soil that develope a close relationship with the roots of host plants[72,73]. They influence plant development, growth, and health via direct or indirect mechanisms, which are even more critical in their establishment and survival under fluctuating climates. Understanding various PGPR-meditated mechanisms that influence plant growth and resilience is critical for their effective field applications as bioinoculants. Resilience against such stresses requires plants to adopt various physiological and molecular strategies, affecting general growth parameters. It is paramount to devise sustainable agricultural practices that enhance the resilience of plants against changing environments without affecting overall growth. The mechanisms of action of PGPR under normal and stressed conditions would be significant for their effective field applications. Moreover, the resilience of PGPR-mediated mechanisms under stressful environments must be comprehensively assessed.
-
PGPR can promote plant growth by controlling phytopathogens, essentially by generating metabolites adding to the antibiosis and antifungal properties utilized as defence activators[74,75]. The beneficial effects on plant growth are more significant when plants are grown in nutrient-poor or stressed soils. Some traits that can indirectly facilitate plant growth include the ability to solubilize phosphate and other minerals, fixation of atmospheric nitrogen, production of siderophores, and 1-aminocyclopropane-1-carboxylate (ACC) deaminases[76]. Changes in agricultural practices modulate the PGPR-induced modifications in the rhizosphere microbial community, affecting plant growth and fitness. However, details are still unclear regarding the interplay of the indigenous microbial community and PGPR in the modifying rhizosphere or bulk soil microbial community and, thus, how it governs plant growth or health (Fig. 2). Biocontrol strains release volatile organic compounds (VOCs) that stimulate plant development, suppress bacterial, fungal, and nematode pathogens, and establish systemic resistance in plants against phytopathogens[77]. VOCs are produced by bacteria from several genera, including Serratia Stenotrophomonas, Pseudomonas, Arthrobacter, and Bacillus, which are reported to influence plant development. The most efficient VOCs for preventing fungal development and increasing plant development are 2,3-butanediol, and acetoin produced by Bacillus sp.[78]. VOCs mediate increased disease resistance, abiotic stress tolerance, and plant biomass from PGPR strains directly or indirectly[79]. Release of VOCs is distinguishing feature of an extensive range of soil microbes and comprising cyclohexane, benzene, decane, 2-(benzyloxy) ethanamine, methyl, dodecane, tetradecane, benzene (1-methylnonadecyl), 1-chlorooctadecane, 2,6,10-trimethyl, and dotriacontane, though the amount and distinctiveness of the VOCs released differ between species[80].
Potential rhizobacteria can ensure modulation of plant-microbe interactions via biochemical mechanisms creating protective environments around and within plant tissues, the induction of plant cellular signaling pathways affecting the growth of the tissue microbiome, and the alteration of plant defence responses towards particular biotic stresses [81,82]. Plant-induced modulation is well understood in general terms, however, many details remain to be elucidated. Because of the variety of PGPR currently being examined, the availability of models from simple to complex systems, and the refinement of analytical approaches, intricate details may soon be resolved[83]. The plant host perceives PGPR as beneficial microbes, although many other roles (neutral and pathogenic) exist within the microbe-plant interaction continuum. Therefore, this group of microbes has adaptations that allow modulation of the plant response to create net benefits. Many plants develop systemic resistance to environmental or biotic stresses when treated with PGPR[84]. Upon pathogen attack, the vascular system produces impulses and activates a defensive mechanism, triggering the release of a large number of defensive enzymes, including chitinase, 1,3-glucanase, polyphenol oxidase, phenylalanine ammonia-lyase, peroxidase, superoxide dismutase, lipoxygenase, and catalase, as well as certain proteinase inhibitors. ISR is not unique to a single pathogen, but it aids plants in managing a range of pathogens[85]. ISR includes ethylene hormone signaling in the plant, which induces host defence against various plant diseases[86]. Even though the majority of PGPR initiates ISR in plants and their use has the potential to revolutionize agriculture, fundamental research on PGPR and the application of existing tools and techniques to help plants from the lab to the field has been rare[87]. The efficiency of PGPR varies by crop; however, particular strains promote growth in various crops. PGPRs are powerful agents for increasing agricultural productivity.
-
Changing climate has a profound impact on plant physiology, influencing the availability and uptake of nutrients either directly or indirectly via soil changes. A comprehensive understanding of plant response to the changing climate and its implications on nutrient dynamics is essential[88]. This understanding is crucial for developing effective management strategies to avoid potential nutrient-related challenges that could undermine food security. It is a consensus among scientists that agricultural practices need to be more sustainable to ensure food security under the changing climate. Shifting agricultural practices involve complex changes in the global systems and ecosystems that influence the feedback relevance of nutrient cycling to the climate. The climate-induced changes in the biogeochemical cycles and their impact on global systems and ecosystems indicate a necessity for a better understanding of plant response to the changing climate, particularly focusing on the changes in nutrient dynamics[89].
Biological nitrogen fixation occurs for the most part at mild temperatures by nitrogen-fixing microorganisms[90], and the nitrogenase complex is an intricate chemical that is a key component in N2 fixation[91]. The nitrogen fixation procedure requires the nitrogenase gene (Nif gene) symbiotic association in the rhizobium is subject to low oxygen concentration, controlled by another cluster of genes called fix genes, which are general for both symbiotic and free-living nitrogen fixation systems[92,93]. The rhizobacteria help plant growth by providing essential nutrients through inorganic phosphate solubilization and iron siderophores production, improving iron and phosphorous uptake[94,95]. PGPR within the soil employs distinctive methodologies for the utilization of inaccessible forms of phosphorus and also helps phosphorus assimilation[96]. Besides providing phosphorus to the plants, the phosphate solubilizing microbes also help develop plants by stimulating the proficiency of biological nitrogen fixation, improving the accessibility of other trace components by producing important plant growth promoting elements[97].
Research on PGPR shows that they improve plant nutrient uptake, encouraging sustainable agriculture. Many PGPR species help acquire nutrients through biological nitrogen fixation, phosphate solubilization, siderophore synthesis for iron chelation, and root system regulation (Table 2). Azospirillum brasilense stimulates rice root growth and fixes atmospheric nitrogen[98,99], while P. fluorescens secretes organic acids to help wheat solubilize phosphorus[100,101]. In soybean roots, Bradyrhizobium japonicum forms nitrogen-fixing nodules that boost nitrogen assimilation[102]. Ammonia and siderophore release by other PGPR strains such as Bacillus subtilis in maize helps absorb nitrogen and iron[103]. Bacillus amyloliquefaciens increases phosphate and calcium availability in lettuce and tomato, whereas Rhizobium etli improves potassium uptake and root system architecture. Endophytic nitrogen fixation and hormone synthesis by Gluconacetobacter diazotrophicus in sugarcane boosts plant development under nutrient-limiting conditions[104]. However, PGPR's efficiency also depends on plant species and environmental conditions. Pseudomonas aeruginosa in bananas uses siderophore activity to solubilize iron and zinc[105]. Growing evidence shows that PGPR reduce chemical fertilizer dependency, improves soil health, and boost crop yield, making them crucial in sustainable agriculture systems[13]. Future research should optimize PGPR treatments in diverse agroecosystems to maximize benefits across crop species and soil conditions.
PGPR can solubilize the potassium rock through organic acid synthesis and release[106]. Hence, the application of potassium solubilizing PGPR as biofertilizer for horticulture improvement can reduce the utilization of agrochemicals and backing ecofriendly crop production[107]. Although iron is the fourth most inexhaustible component on earth[108], it is just sparingly dissolvable that the measure of iron accessible for assimilation by living organisms is very low[109]. Both microbes and plants require a significant level of iron, and acquiring adequate iron is considerably more difficult within the rhizosphere, where parasites, microbes, and plants compete for iron[110]. To deal with such a restricted supply of iron, PGPR improves the iron availability through synthesizing siderophore[111]. The soil fertility, plant health, and productivity are interconnected. The health of the soil depends on the biological processes and availability of nutrients[112]. The soil enriched with organic amendments results in an increase in the microbial population, diversity, and microbial-induced biochemical activities, followed by the mineralization of nutrients to enhance plant growth and productivity[113,114]. On the other hand, the organic amendments induce changes in the microbial community that increase plant disease susceptibility as diseases lower plant growth and productivity by affecting plant health.
Table 2. PGPR strains, targeted nutrients, and their specific mechanisms of action.
Crop PGPR species Nutrient enhanced Mechanism of action Ref. Rice (Oryza sativa) Azospirillum brasilense Nitrogen (N) Biological nitrogen fixation (BNF), production of auxins stimulating root growth [98,99] Wheat (Triticum aestivum) Pseudomonas fluorescens Phosphorus (P) Phosphate solubilization via organic acid production [100,101] Maize (Zea mays) Bacillus subtilis Nitrogen (N), Iron (Fe) Siderophore production for iron chelation, ammonia production for nitrogen supply [103] Soybean (Glycine max) Bradyrhizobium japonicum Nitrogen (N) Symbiotic nitrogen fixation in root nodules [102] Tomato
(Solanum lycopersicum)Rhizobium etli Potassium (K), Phosphorus (P) Enhancement of root architecture, potassium and phosphate solubilization [115] Barley (Hordeum vulgare) Paenibacillus polymyxa Phosphorus (P), Zinc (Zn) Phosphate solubilization and zinc mobilization [116] Peanut (Arachis hypogaea) Rhizobium sp. Nitrogen (N), Phosphorus (P) Rhizosphere colonization promoting nutrient uptake [117] Sugarcane
(Saccharum officinarum)Gluconacetobacter diazotrophicus Nitrogen (N) Endophytic nitrogen fixation and hormone production [104] Potato
(Solanum tuberosum)Azotobacter chroococcum Nitrogen (N), Phosphorus (P) Nitrogen fixation and phosphatase enzyme production [118] Lettuce (Lactuca sativa) Bacillus amyloliquefaciens Calcium (Ca), Phosphorus (P) Solubilization of calcium and phosphorus, root growth promotion [50] Carrot (Daucus carota) Pseudomonas putida Phosphorus (P), Potassium (K) Mineral solubilization, root elongation stimulation [119] Chickpea (Cicer arietinum) Mesorhizobium ciceri Nitrogen (N) Formation of root nodules, nitrogen fixation [120] Cotton
(Gossypium hirsutum)Bacillus megaterium Phosphorus (P), Sulfur (S) Phosphate solubilization and sulfur oxidation improving plant growth [121] Sunflower
(Helianthus annuus)Azotobacter vinelandii Nitrogen (N), Phosphorus (P) Nitrogen fixation and mobilization of phosphorus in the rhizosphere [122] Banana (Musa spp.) Pseudomonas aeruginosa Iron (Fe), Zinc (Zn) Siderophore-mediated iron uptake, zinc solubilization [105] Apple (Malus domestica) Bacillus thuringiensis Phosphorus (P), Nitrogen (N) Phosphate solubilization, nitrogen fixation [123] Strawberry
(Fragaria × ananassa)Pseudomonas stutzeri Iron (Fe), Calcium (Ca) Iron chelation through siderophore production, calcium mobilization [124] Grapes (Vitis vinifera) Bacillus velezensis Magnesium (Mg), Phosphorus (P) Root enhancement, improved magnesium uptake [125] Pepper
(Capsicum annuum)Pseudomonas fluorescens Nitrogen (N), Sulfur (S) Sulfur oxidation, nitrogen fixation, biocontrol properties [126] -
PGPR produces phytohormones, called plant growth regulators (PGRs)[127,128], that might be integrated into defined organs of plants and can be transported to different sites, where these trigger explicit morphological, physiological, and biochemical parts in plant growth development and improvement[95]. They are utilized as plant growth regulators and are potential options for increasing agriculture production. One of the prominent modes of action by PGPR for plant growth promotion is the production of a plant growth regulator or phytostimulator[129]. This is characterized by microorganisms that can produce or change the growth regulator concentration, for example, indole acetic acid (IAA), ethylene, gibberellic acid (GA), and cytokinin. The phytohormones, such as GA, auxins, and cytokinin, regulate plant growth and development and subsequently, other PGPR were found to produce IAA, the most widely studied phytohormone. The biochemical pathways for the biosynthesis of IAA and other phytohormones in PGPR shed light on the evolution of these pathways that plant hormones have co-evolved in rhizobacteria as adaptations to promote plant growth, an ancestral trait that has been independently examined by species of PGPR across taxonomic groups[130−132]. Furthermore, in addition to biotic and abiotic signals, PGPR imparts a variety of chemical signals that elicit plant growth or defense responses (Fig. 3). An extensive range of root-secreted and exuded chemical signals from plants, including organic acids, phenolics, flavonoids, terpenes, mucilage, sugars, and amino acids, could influence microbial community structure and activity. Understanding these complex signaling mechanisms could provide critical knowledge for the sustainable use of PGPR in agricultural practices. Furthermore, this understanding could profoundly impact designing synthetic microbes that could elicit specific plant responses in a desired manner[133−135].
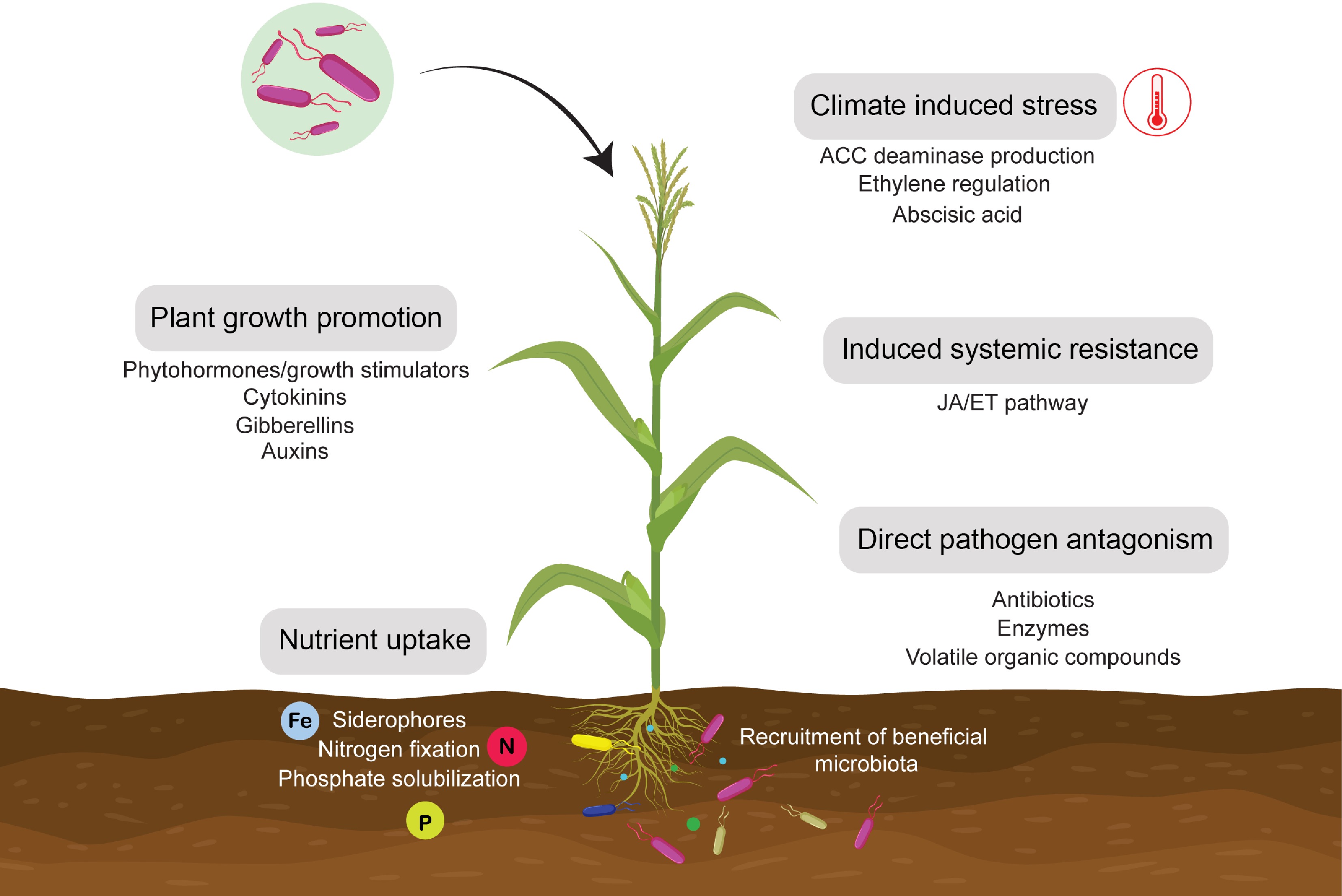
Figure 3.
Plant growth promoting and disease resistance mechanisms of PGPR.
PGPR produce or modulate plant growth promoting hormones such as cytokinin, auxins and gibberellins, as well as improves nutrient uptake and assimilation through nitrogen fixation, phosphorus solubilization and siderophore production. PGPR also reduce toxic ethylene levels through ACC deaminase production and regulating stress related hormones. PGPR directly antagonize pathogens by producing antibiotics, enzymes and volatile organic compounds or indirectly through induced systemic resistance which is achieved by activation of JA/ET pathway against pathogen attack.Further, more than 30 separate growth promoting cytokinin compounds have been discovered in plants, plant-associated microbes, and in vitro environments, where most microbes can produce cytokinin in varying proportions[136−138]. Cytokinin synthesis has been studied in a variety of plant-associated microbes. These bacteria belonging to the genera Bacillus, Pseudomonas, and Azospirillum were isolated from various plant species, including barley, canola, bean, and Arabidopsis[139]. In addition, gibberellins are a family of phytohormones that include up to 136 organized molecules. This group of phytohormones influences seedling growth, stem elongation, budding, and fruit development. PGPR with ACC improves crop development and yield[42,140], and its role in producing beta-glucanase, phosphatase, dehydrogenase, and antibiotic agents has also received attention[141]. The evolutionary co-dynamics of plants and PGPR under key mechanisms involve plant growth-promoting phytohormones or traits across diverse groups of bacteria. There is growing interest in the role of phytohormones in the plant growth-promoting and biocontrol activities of these bacteria. Changes in root morphology and architecture promote the establishment of beneficial interactions between plants and microorganisms in the soil, enhancing nutrient uptake and pathogen resistance.
-
The valuable employment of potential rhizobacteria for potential functions has explicit positive effects on crop efficiency and agriculture sustainability. Therefore, their useful implementation should be widely adopted in the fundamental agriculture framework. This is the demand of the climate change era to create awareness among the crop producers about the potential advantages where utilizing PGPR as opposed to cost ineffectual methodologies based on chemical fertilizers. The current studies on the impact of climate change on plants and PGPR emphasize co-evolutionary dynamics in host plants and the urgent requirement for sustainable agricultural methods. The presence of these rhizobacteria is crucial for the growth and development of plants as they improve the availability of nutrients and increase the plant's ability to withstand stress. However, climate change directly impacts PGPR population and potential activity, disrupting their interaction with ecosystem services. There is a need to prioritize the advancement of climate-specific agricultural methodologies and the assessment of the intricate interplay between PGPR and soil health, as well as the impact of changing surroundings on PGPR. Additionally, it is necessary to investigate the exploration of novel PGPR species and their potential long-term applicability in the context of climate change.
PGPR holds transformative potential in addressing climate challenges through both natural and engineered mechanisms. Genetic advancements could tailor strains for precision adaptation e.g., ACC deaminase-overexpressing PGPR to combat drought or heat-tolerant variants engineered with extremophile genes. Such strains could reduce reliance on synthetic fertilizers, cutting agricultural green house gase emissions (30%–50% lower N₂O in rice fields using Azospirillum biofertilizers) while enhancing carbon sequestration via root-driven soil organic matter stabilization. Interdisciplinary integration pairing synthetic biology with artificial intelligence-driven soil sensors could optimize PGPR deployment in real-time, predicting plant-microbe interactions under shifting climates. Beyond crops, PGPR may aid ecosystem restoration, such as detoxifying heavy metals in degraded soils or reversing desertification via seed-coating technologies. However, scaling these solutions requires overcoming biosafety concerns (CRISPR-based 'kill switches' to control engineered strains) and socio-technical barriers, including farmer acceptance and equitable policy frameworks. Global collaboration, through initiatives like COP28-funded trials or open-access genomic databases, will be critical to unlocking the role of PGPR in sustainable intensification. By bridging agroecology, biotechnology, and circular economy principles, PGPR could emerge as cornerstones of climate-smart agriculture, ensuring food security while mitigating planetary crises.
This research was financially supported by the Yunnan Agricultural University Startup Fund (20232020003).
-
The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
-
The author declares that there is no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Yunnan Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Munir S. 2025. Evolution of plant growth promoting rhizobacteria under global climate change. Agrobiodiversity 2(2): 33−43 doi: 10.48130/abd-0025-0005
Evolution of plant growth promoting rhizobacteria under global climate change
- Received: 01 January 2025
- Revised: 05 April 2025
- Accepted: 16 April 2025
- Published online: 19 May 2025
Abstract: The advancement of life in all its forms relies on agricultural practices and food security, which are fundamentally influenced by soil characteristics. The demand for agricultural products has surged due to climate change and global population growth, leading to a significant increase in the utilization of chemical fertilizers. Since chemical agents have affected soil fertility and quality by degradation, causing the development of agricultural fields with rich soil to be incomprehensible, consideration for a secure and profitable agricultural production has drifted. Plant growth promoting rhizobacteria (PGPR) have established eco-evolutionary relationships with host plants, fitness, and productivity. Microbial renaissance driven by plant growth regulators has been successfully achieved through disease resistance in emerging pathogens, bio-fertilization, rhizoremediation, and stimulating root growth. PGPR can profoundly increase plant growth by producing growth hormones, improving nutrient uptake, suppressing plant pathogens, and protecting against biotic and abiotic stress conditions. Here, we provide insight into sustainable agricultural development aimed at increasing crop yield and production by exploring the mechanisms and commercial applications of potential rhizobacteria under changing climatic conditions.
-
Key words:
- Climate change /
- Emerging pathogen /
- Disease /
- Mutualism /
- Microbial evolution












