-
Intrauterine growth retardation (IUGR) refers to the phenomenon that the embryo/foetus fails to reach its expected growth potential, and it is the most common pregnancy complication for animal production[1]. Among livestock species, pigs exhibit the most severe natural occurrence of IUGR, with an incidence of 15% to 20%[2]. The liver is a crucial metabolic, immune, and secretory organ, playing an important role in stimulating systemic growth and improving disease resistance[3]. Numerous studies have shown that the liver is a target organ affected by IUGR, and its structure and function are severely compromised in the postnatal stage, in part contributing to high morbidity and mortality, as well as stunted growth. For example, Zhang et al. reported that IUGR caused hepatic oxidative damage and hepatocytes apoptosis by increasing the accumulation of superoxide radicals and oxidative damage products, impairing hepatic and mitochondrial superoxide dismutase (SOD) activity, and disrupting mitochondrial biogenesis and energy homeostasis[4,5]. Additionally, translation and transcriptional expression of hepatic cytokines, such as interleukin 1 beta (IL1β), interleukin 6, and tumor necrosis factor alpha, were upregulated, indicating that hepatic inflammation occurred in IUGR weaned piglets[6]. IUGR also induced hepatic lipid metabolism disorder, evidenced by the increased non-esterified fatty acid concentration, which may be a cause of oxidative stress[7]. Furthermore, the abnormal liver function index in the serum (e.g., alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), coupled with hepatic pathological and ultrastructural morphological changes, corroborated liver damage and dysfunction in IUGR piglets[4,8]. In addition, liver transcriptome profiling and functional analysis of IUGR piglets reveal that the livers of IUGR piglets were associated with a series of complications, including inflammatory stress and immune dysregulation, cytoskeleton and membrane structure disorganization, dysregulated transcription events, and abnormal glucocorticoid metabolism[8]. Therefore, the potential mechanisms underlying IUGR-induced hepatic damage in piglets include oxidative stress, mitochondrial dysfunction, and overproduction of pro-inflammatory cytokines. Regulating antioxidant capacity, inflammation, and mitochondrial function may be a promising strategy for attenuating hepatic damage in IUGR piglets.
Resveratrol (RSV) is a natural polyphenol that is widely present in peanuts, grapes, and mulberries. RSV exhibits multiple biological activities such as antioxidant, anti-inflammatory, antibacterial, and antiviral effects. Studies conducted in animal or in vitro models demonstrated that RSV can prevent and/or slow the onset and development of a wide variety of liver diseases, including liver fibrosis[9], cholestatic liver damage[10], chemical liver injury[11], and hepatocellular carcinoma and metastasis[12], which is mainly related to its antioxidant activity[13]. In addition, RSV has also been incorporated into diet as a potential feed additive in poultry and livestock. As for broilers, dietary RSV supplementation alleviates hepatic oxidative damage by activating nuclear factor-like 2/kelch-like ECH-associated protein 1 signaling pathway to improve growth performance under heat stress[14]. Similar liver protection effects of RSV were observed in broilers exposed to lipopolysaccharide[15] and aflatoxin B1[16]. Recently, maternal RSV supplementation has been reported to increase catalase and T-SOD activities in the liver of suckling piglets under oxidized soybean oil challenge[17]. A previous study showed that RSV improved liver damage in IUGR finishing pigs through enhancing mitochondrial function, regulating immune and redox status, and inhibiting inflammation[18]. In suckling piglets, RSV has also been demonstrated to effectively attenuate IUGR-induced mitochondrial dysfunction in the liver by improving mitochondrial biogenesis and antioxidant ability[4]. However, little is known about the protective effects of RSV on liver damage in weaned piglets resulting from IUGR. Moreover, as a defensive function in the advancement of liver diseases, the role of mitophagy in the hepatoprotective effects of RSV on IUGR piglets is still unclear. Therefore, in this study, the effect of RSV on redox status, inflammation, mitochondrial function, and mitophagy in the liver of IUGR weaned piglets was investigated, which could provide a theoretical basis for future rational utilization of RSV in swine production.
-
At birth, 12 male normal birth weight piglets (Duroc × Landrace × Yorkshire, ~1.59 kg), and 24 same-sex IUGR piglets (Duroc × Landrace × Yorkshire, ~0.94 kg) were chosen from 12 sows that met the selection criteria for IUGR[18]. Piglets were allowed to suck the dam naturally up to 26 d of age. After that, from 26–47 d of age, all piglets were completely weaned and randomly assigned to three groups based on body weight. Each group consisted of six replicates (pens, 2 m × 0.6 m) with two piglets per replicate. In detail, the NC group, NBW piglets fed a basal diet; the IC group, IUGR piglets fed a basal diet; the IR group, IUGR piglets fed a basal diet supplemented with 300 mg/kg RSV. The dosage of RSV used in this study was selected based on a previous study, which showed that feeding weaned piglets a diet containing 300 mg/kg of RSV was effective in preventing deoxynivalenol-induced oxidative stress[19]. Resveratrol (purity, 98%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All weaned piglets were housed in a controlled temperature (26 ± 2 °C) and humidity (60% ± 5%) room with natural lighting and had free access to feed and drinking water. The composition and nutrient levels of the basal diet are listed in Supplementary Table S1 and meet the National Research Council (2012) nutritional requirements standards[20].
Sample collection
-
At the end of this trial, one piglet per replicate was selected and weighed after overnight fasting. Blood samples were collected from the anterior vena cava, and then the piglets were euthanized after anesthesia. The entire liver sample was collected and weighed. A fraction of the chopped liver sample from the same left lobe was snap-frozen in liquid nitrogen. Serum was obtained by centrifuging at 2,000 × g for 20 min at 4 °C. Collected samples were stored at –80 °C for analysis.
Analysis of serum aminotransferase activities
-
Serum ALT (catalog no. C009-1-1) and AST (catalog no. C010-1-1) activities were measured by commercial kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China), according to the manufacturer's recommendations.
Hepatic redox status analysis
-
Hepatic redox status was evaluated by monitoring changes in T-SOD (catalog no. A001-1-2) and glutathione peroxidase (GPX, catalog no. A005-1-2) activities, malondialdehyde (MDA, catalog no. A003-1) and reduced glutathione (GSH, catalog no. A006-1-1) levels, which were measured using commercial kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China), according to the manufacturer's protocols.
Hepatic cytokines contents analysis
-
The ELISA kits (Hongsheng Biotechnology Co., Ltd., Nanjing, China) were adopted to measure hepatic interleukin 18 (IL18, catalog no. E71041), and IL1β (catalog no. E78620) concentrations.
Hepatic adenosine triphosphate (ATP), and mitochondrial electron transport chain complexes assay
-
Commercial ELISA kits (Jiangsu Meimian Industrial Co. Ltd., Yancheng, China) were used for the determination of hepatic ATP (catalog no. MM-32675O1), mitochondrial respiratory chain complex I (catalog no. MM-7758101), III (catalog no. MM-7757101), and V (catalog no. MM-7757301) contents.
Relative mRNA expression
-
Total RNA in liver was extracted by the Total RNA Isolation Reagent (catalog no. R401-01, Vazyme, Nanjing, China) and reverse transcribed into cDNA using HiScript II Q RT Select SuperMix for qPCR(+gDNA wiper) (catalog no. R223-01, Vazyme, Nanjing, China) according to the manufacturer's instructions. The qRT-PCR analysis was performed using ChamQ SYBR qPCR Master Mix (catalog no. Q711-02, Vazyme, Nanjing, China) following the recommended process of the manufacturer. The primers are listed in Supplementary Table S2 and manufactured by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Relative mRNA expression was assessed using the 2−ΔΔCt method with glyceraldehyde-3-phosphate dehydrogenase as the reference gene.
Statistical analysis
-
Statistical analysis were performed using SPSS statistical software (SPSS version 27.0; SPSS, Inc., Chicago, IL, USA). Statistical differences were determined by one-way analysis of variance and Tukey's post hoc test for multiple comparisons test. Statistical significance level for all analysis was set at p < 0.05. Data are presented as means and standard errors.
-
Compared with the NBW, IUGR reduced (p < 0.05) the absolute weight of the liver by 20.67% (Fig. 1a) and increased serum AST activity by 81.14% (Fig. 1c) in piglets. RSV did not alter the absolute weight of the liver (p > 0.05) in the IR group, and this index was lower than that in the NC group (p < 0.05). In contrast, RSV reversed the elevated serum AST activity in comparison with the IC group (p < 0.05), with its value being statistically equivalent to that of the NC group (p > 0.05).
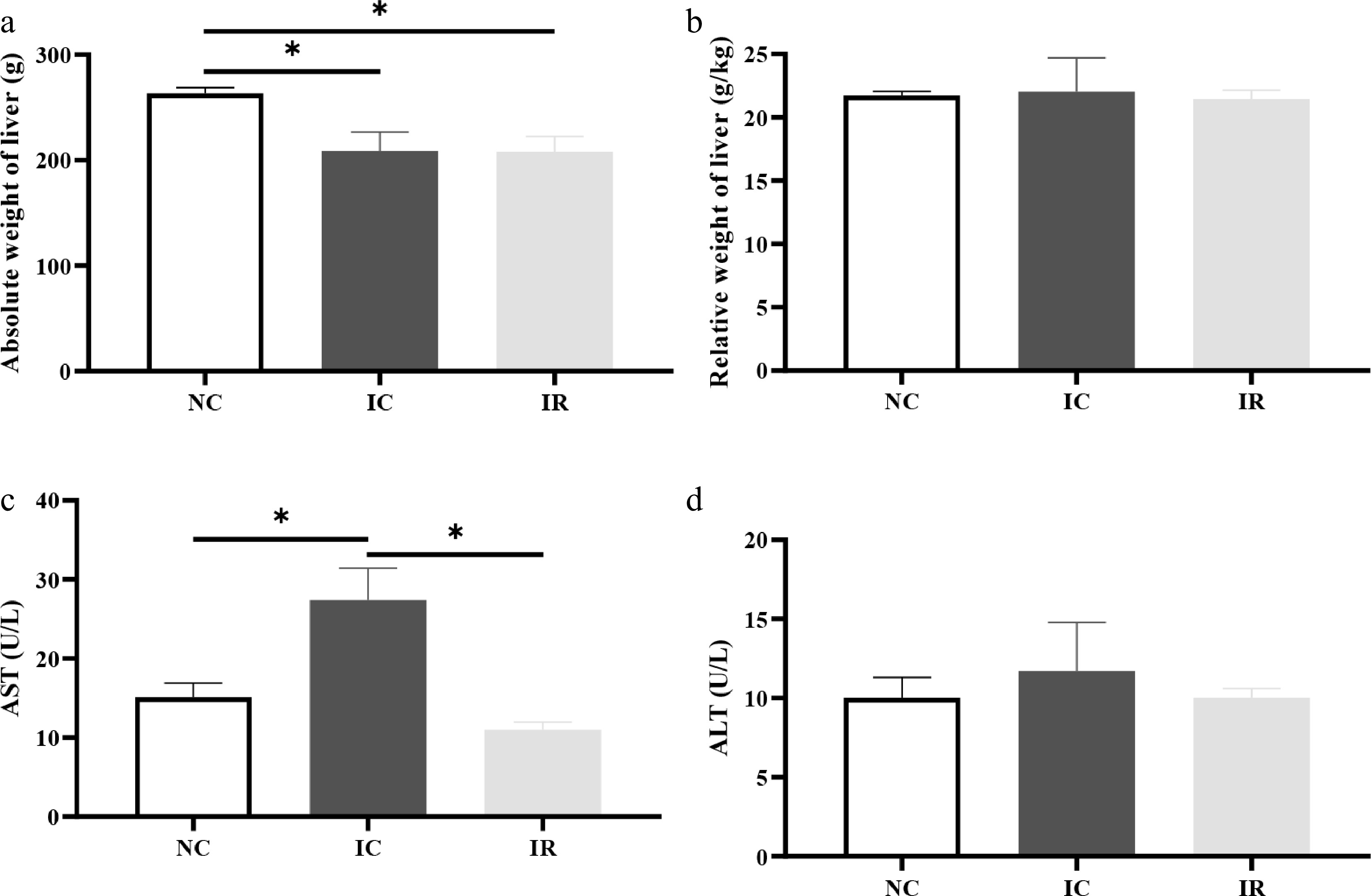
Figure 1.
Resveratrol alleviates liver damage in weaned piglets with intrauterine growth retardation. (a) Absolute weight of liver, (b) relative weight of liver, (c) serum aspartate aminotransferase (AST) activity, (d) serum alanine aminotransferase (ALT) activity. NC, piglets with normal birth weight fed a basal diet; IC, piglets with intrauterine growth retardation fed a basal diet; IR, piglets with intrauterine growth retardation fed a basal diet supplemented with 300 mg/kg RSV. Results are presented as means and standard errors. * p < 0.05.
Hepatic redox status
-
Compared with the NBW (Fig. 2b), IUGR resulted in a 22.57% decrease in hepatic T-SOD activity (p < 0.05). In contrast, supplementing RSV restored the reduced liver T-SOD activity (p < 0.05) and reduced MDA level (Fig. 2a, p = 0.052) in comparison with the IUGR piglets fed a basal diet, with its value being statistically equivalent to that of the NC group (p > 0.05).
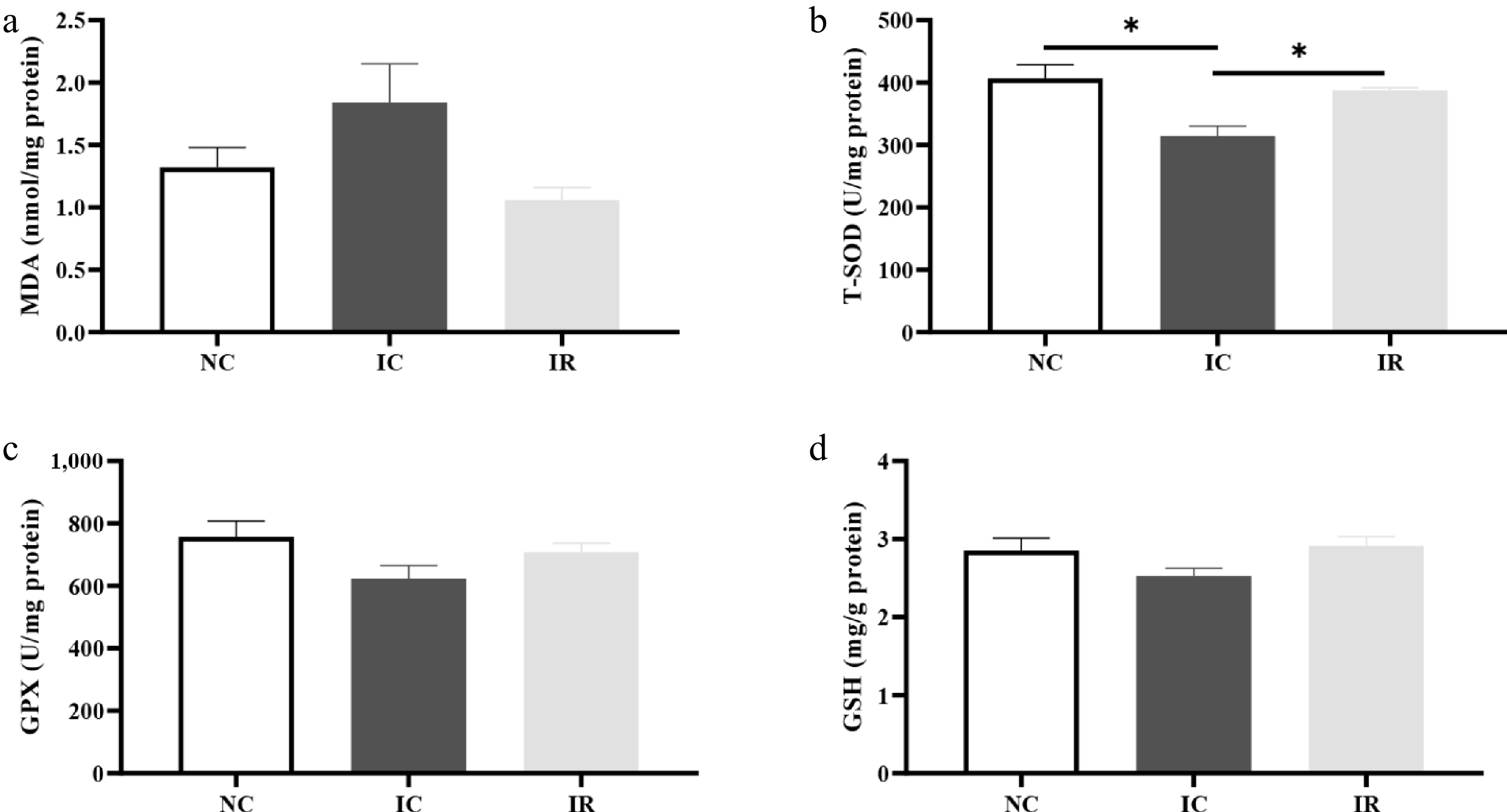
Figure 2.
Resveratrol improves hepatic redox status in weaned piglets with intrauterine growth retardation. (a) MDA, malondialdehyde. (b) T-SOD, total superoxide dismutase. (c) GPX, glutathione peroxidase. (d) GSH, reduced form of glutathione. NC, piglets with normal birth weight fed a basal diet; IC, piglets with intrauterine growth retardation fed a basal diet; IR, piglets with intrauterine growth retardation fed a basal diet supplemented with 300 mg/kg RSV. Results are presented as means and standard errors. * p < 0.05.
IUGR downregulated the mRNA expression of hepatic glutamate-cysteine ligase modifier subunit (Fig. 3a, GCLM, p < 0.05), glutamate-cysteine ligase catalytic subunit (Fig. 3b, GCLC, p < 0.05), SOD1 (Fig. 3c, p < 0.05), and GPX4 (Fig. 3f, p = 0.059) in comparison with the NBW, which were not improved by dietary supplementation with RSV (p > 0.05). Moreover, the values of these aforementioned indices in the IR group were all lower than those in the NC group (p < 0.05), except for GPX4. RSV upregulated hepatic GPX1 mRNA expression in the IR group compared with the IC group (Fig. 3e, p < 0.05).
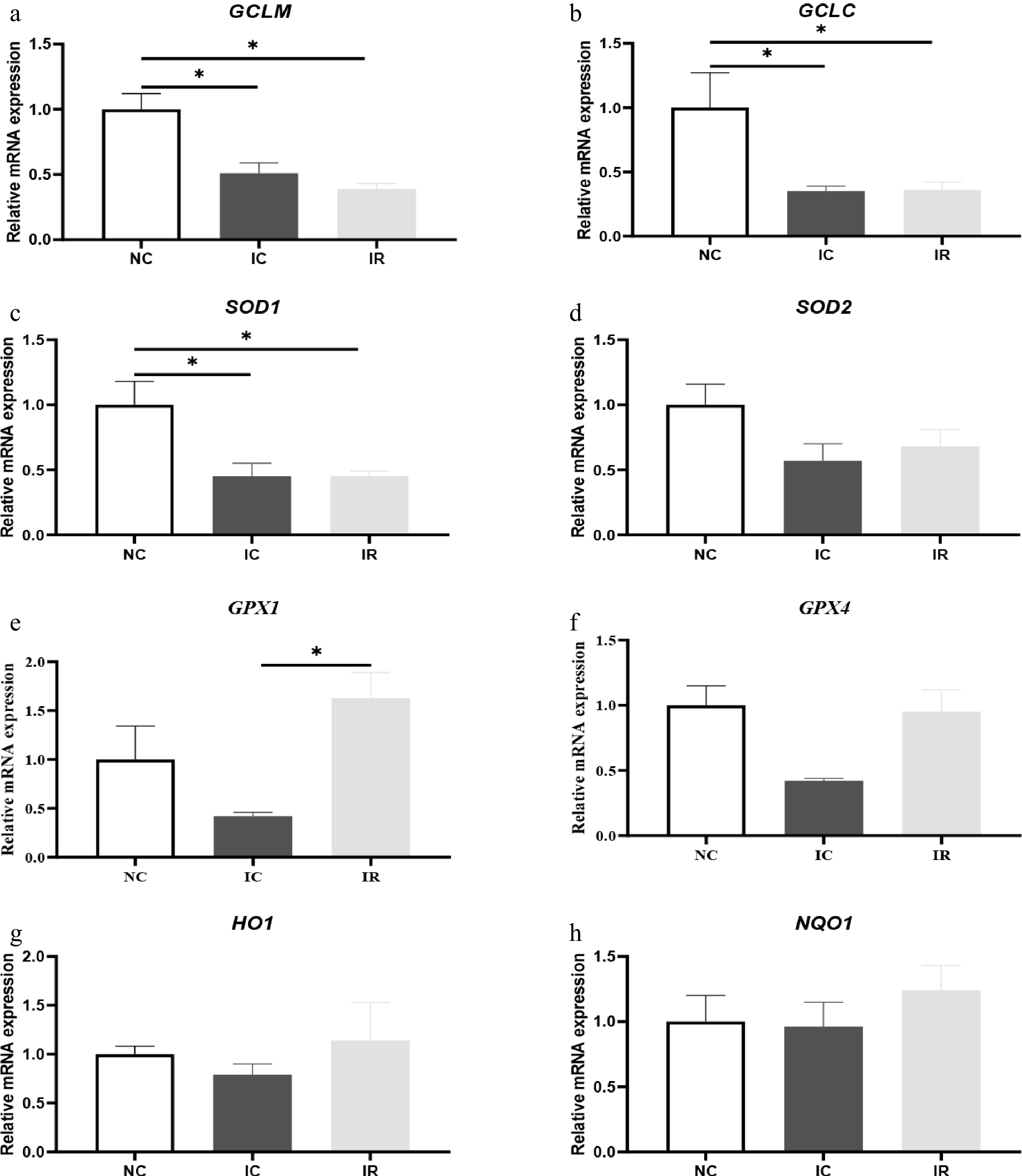
Figure 3.
Resveratrol affects hepatic antioxidant function-related gene expression in weaned piglets with intrauterine growth retardation. (a) GCLM, glutamate-cysteine ligase modifier subunit. (b) GCLC, glutamate-cysteine ligase catalytic subunit. (c) SOD1, superoxide dismutase 1. (d) SOD2, superoxide dismutase 2. (e) GPX1, glutathione peroxidase 1. (f) GPX4, glutathione peroxidase 4. (g) HO1, heme oxygenase 1. (h) NQO1, NAD (P) H: quinone oxoreductase 1. NC, piglets with normal birth weight fed a basal diet; IC, piglets with intrauterine growth retardation fed a basal diet; IR, piglets with intrauterine growth retardation fed a basal diet supplemented with 300 mg/kg RSV. Results are presented as means and standard errors. * p < 0.05.
Hepatic inflammatory response
-
The IUGR-induced increases in hepatic IL18 (Fig. 4a) and IL1β (Fig. 4b) levels were restored by RSV (p < 0.05). Hepatic IL1β content in the NC group was lower than that in the IR group (p < 0.05), while hepatic IL18 content was comparable between the NC and IR groups (p > 0.05).
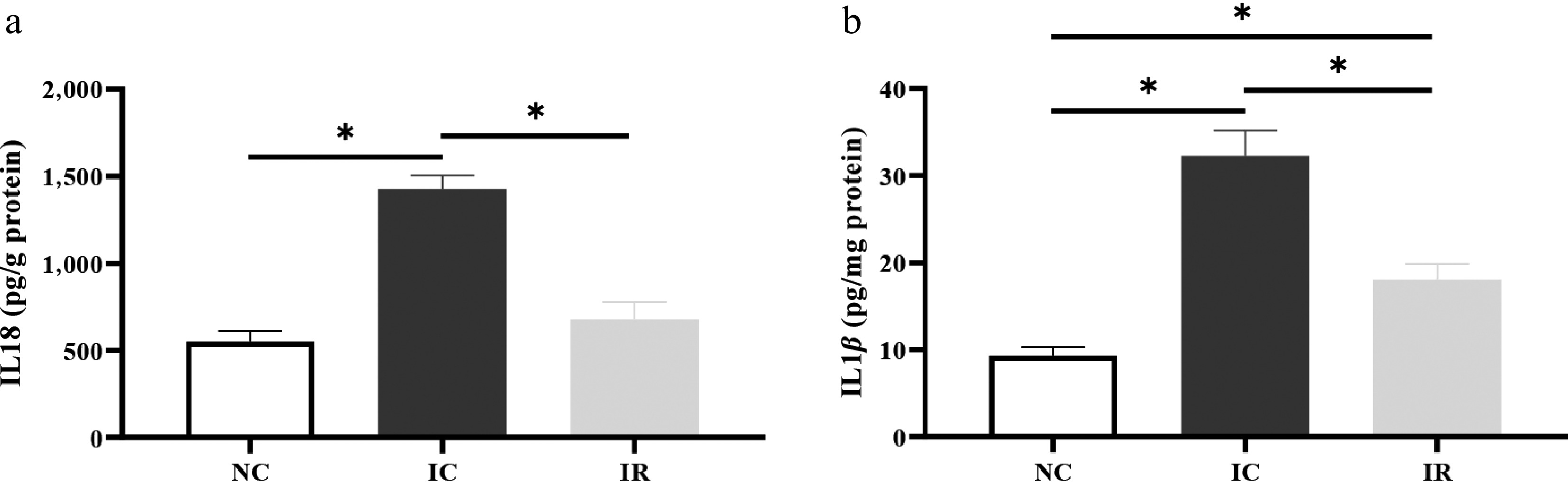
Figure 4.
Resveratrol inhibits hepatic inflammation in weaned piglets with intrauterine growth retardation. (a) IL18, interleukin 18. (b) IL1β, interleukin 1 beta. NC, piglets with normal birth weight fed a basal diet; IC, piglets with intrauterine growth retardation fed a basal diet; IR, piglets with intrauterine growth retardation fed a basal diet supplemented with 300 mg/kg RSV. Results are presented as means and standard errors. * p < 0.05.
Hepatic energy metabolism
-
Compared with the NBW, IUGR resulted in a 56.81% and 21.64% decrease in hepatic ATP (Fig. 5a) and complex I (Fig. 5b) concentrations in piglets (p < 0.05), respectively. In contrast, supplementing RSV significantly increased hepatic ATP content (p < 0.05) in IUGR piglets, but its value was still lower than that of the NC group (p < 0.05). RSV incorporation numerically increased hepatic complex I level in IUGR piglets (p > 0.05), with the value in the IR group being statistically similar to that in the NC group (p > 0.05).
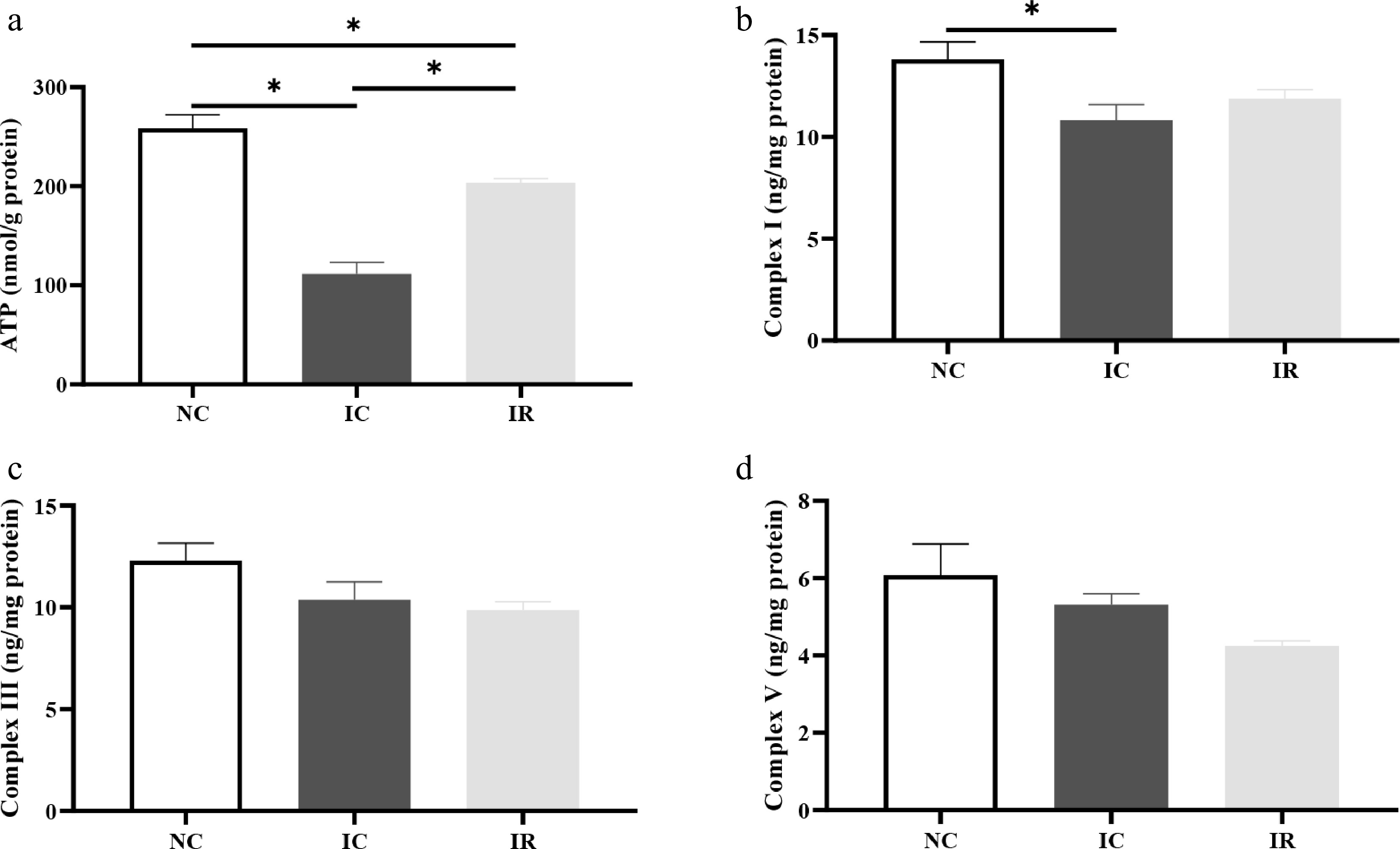
Figure 5.
Resveratrol enhances hepatic energy production in weaned piglets with intrauterine growth retardation. (a) ATP, adenosine triphosphate. (b) Complex I. (c) Complex III. (d) Complex V. NC, piglets with normal birth weight fed a basal diet; IC, piglets with intrauterine growth retardation fed a basal diet; IR, piglets with intrauterine growth retardation fed a basal diet supplemented with 300 mg/kg RSV. Results are presented as means and standard errors. * p < 0.05.
Hepatic gene expression involved in mitophagy
-
When compared with the NC group (Fig. 6e), IUGR increased hepatic BCL2/adenovirus E1B 19-kDa-interacting protein 3-like (BNIP3L) mRNA expression by 118.00% (p < 0.05). RSV supplementation reduced hepatic BNIP3L mRNA expression in the IUGR piglets (p = 0.086), with its value being similar to that in the NBW piglets (p > 0.05). The NBW piglets exhibited higher hepatic BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 mRNA expression than their IUGR counterparts given a RSV-supplemented diet (p < 0.05, Fig. 6d), and the values of this aforementioned parameter in the IR group were lower than the IC group (p = 0.054).
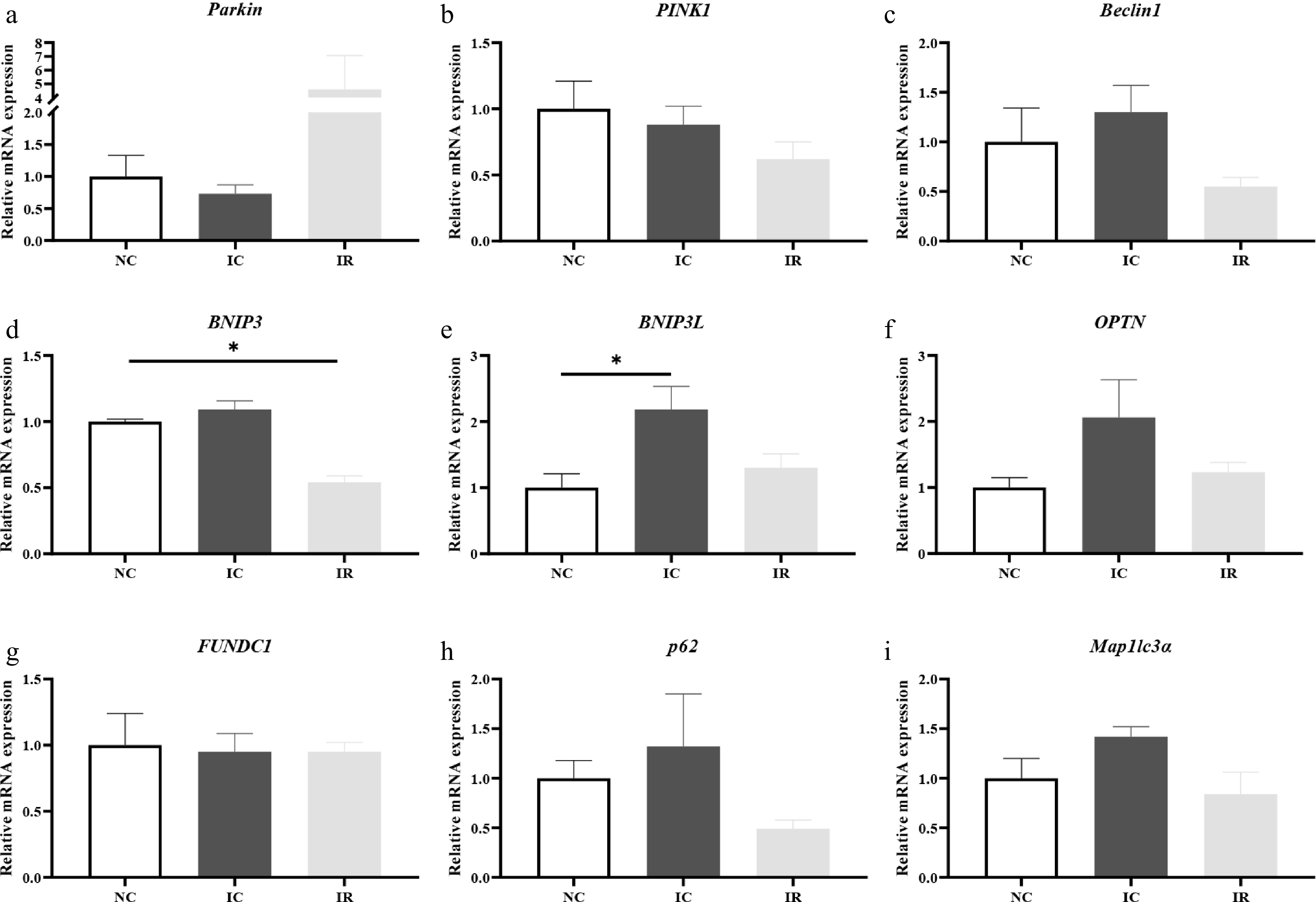
Figure 6.
Resveratrol regulates hepatic mitophagy-related gene expression in weaned piglets with intrauterine growth retardation. (a) Parkin. (b) PINK1, PTEN-induced putative kinase 1. (c) Beclin1. (d) BNIP3, BCL2/adenovirus E1B 19 kDa protein-interacting protein 3. (e) BNIP3L, BCL2/adenovirus E1B 19-kDa-interacting protein 3-like. (f) OPTN, optineurin. (g) FUNDC1, FUN14 domain-containing 1. (h) p62. (i) Map1lc3α, microtubule-associated protein 1 light chain 3 alpha. NC, piglets with normal birth weight fed a basal diet; IC, piglets with intrauterine growth retardation fed a basal diet; IR, piglets with intrauterine growth retardation fed a basal diet supplemented with 300 mg/kg RSV. Results are presented as means and standard errors. * p < 0.05.
-
It was previously reported that the body weight (BW: NC, 11.88 ± 0.07 kg vs IC, 9.41 ± 0.22 kg) at 47 d of age and body weight gain (BWG: NC, 4.16 ± 0.09 kg vs IC: 3.48 ± 0.26 kg) from 26 to 47 d of age were lower in the IUGR piglets than those in the NBW piglets[21]. However, the inferior growth performance of IUGR piglets was not improved after RSV supplementation (BW: IC, 9.41 ± 0.22 kg vs IR, 9.27 ± 0.30 kg; BWG: IC, 3.48 ± 0.26 kg vs IR, 3.48 ± 0.18 kg). Liver injury in IUGR piglets is mainly caused by an imbalance of redox, immune, and mitochondrial homeostasis, contributing to postnatal high morbidity and mortality, and stunted growth. This study presents that RSV protects IUGR piglets against liver injury, possibly by enhancing antioxidant capacity, inhibiting inflammation, and improving mitochondrial oxidative phosphorylation (OXPHOS) and mitophagy-related gene expression.
Previous studies have reported that IUGR decreases the levels of antioxidants and renders hepatocytes more susceptible to free radical-induced injury[4,5]. An important antioxidant affected by IUGR is SOD. SOD acts as the first line of defence against reactive oxygen species (ROS) and catalyses the conversion of superoxide anion free radicals to molecular oxygen and hydrogen peroxide in the cells[22]. In the present study, IUGR depleted hepatic T-SOD activity partly by down-regulating SOD1 mRNA expression. In addition, IUGR piglets exhibited lower hepatic GPX4, GCLM, and GCLC mRNA abundance. These results suggested that IUGR impaired the hepatic antioxidant defense system in piglets. Cheng et al. found that RSV could attenuate increased MDA levels in the liver of IUGR finishing pigs by enhancing T-SOD and glutathione reductase activities[18]. As expected, results showed that RSV supplementation could reverse the negative effect of IUGR on hepatic redox status, evidenced by increased T-SOD activity and reduced MDA level, which was partly attributed to the reduced production of ROS. The main mechanism may be related to the following aspects: (1) hydroxyl groups at 4' and 5' positions in RSV have the potential to eliminate free radicals (e.g., superoxide anion and hydrogen peroxide)[23]. (2) RSV supplementation alleviated IUGR-induced decreased complex I activity, which aids in the reduced production of superoxide radicals from the mitochondrial respiratory chain. Interestingly, the beneficial effects of RSV on hepatic T-SOD activity in IUGR piglets were independent of its regulation of gene expression. In addition to the transcriptional expression, which needs to be further studied. Collectively, these beneficial effects could contribute to the amelioration of liver injury in IUGR piglets.
Increased inflammatory response associated with IUGR plays an important role in liver damage. Elevated hepatic pro-inflammatory cytokine production has been found in the IUGR rat[24] and pig model[18]. Consistent with these previous studies, increased levels of hepatic IL18 and IL1β in IUGR weaned piglets were also observed, indicating that inflammation occurred and may lead to liver injury in IUGR piglets. Multiple evidence from laboratory studies, both in vivo and in vitro, reported that the anti-inflammatory properties of RSV were associated with the inhibition of pro-inflammatory factors production through regulating a number of signaling pathways, including nuclear factor kappa B[25], mitogen-activated protein kinase[26,27], and arachidonic acid[28]. It was also found that feeding an RSV-supplemented diet alleviated hepatic inflammation in IUGR piglets, as evidenced by suppressed IL18 and IL1β levels. This partly contributed to the recovery of liver damage in the IUGR piglets. In domestic animals such as broilers, ducks, and pigs, RSV has also been documented to be able to protect against inflammatory damage caused by heat stress[29], lipopolysaccharide[30], deoxynivalenol[31], and aflatoxin B1[32].
Mitochondria are well-known organelles that provide energy for eukaryotic cells. OXPHOS, composed of five multimeric enzymes in the mitochondrial electron transport chain, drives ATP synthesis[33]. In addition, approximately 90% of ROS are produced by OXPHOS in mitochondria[34]. Mitochondrial OXPHOS disorders lead to excessive ROS production, which can damage cellular biomacromolecules (i.e., lipids, proteins, and nucleic acids) and ultimately lead to cellular apoptosis. Mitochondria are abundant in hepatocytes, with each hepatocyte containing about 800 mitochondria. Mitochondrial OXPHOS disorders have been demonstrated to exert an important role in the development of liver damage induced by various insults, including IUGR[4,18]. Similarly, results showed that IUGR inhibited hepatic complex I and ATP concentrations in piglets. In addition, mitophagy-related to signaling molecule expression was up-regulated in the liver of IUGR piglets. Mitophagy is a selective macroautophagy that facilitates the removal of ROS-producing mitochondria and ultimately leads to the degradation of mitochondria in the lysosome[35]. In mitophagy, an important regulatory pathway is mediated by mitophagy receptors, one of which is BNIP3L. BNIP3L is a mitochondrial protein from the outer membrane that promotes mitophagy either by recruiting autophagosomes or by enhancing the formation of autophagosomes[36]. In this study, the increased BNIP3L expression in the liver of IUGR piglets may be considered as a protective adaptive mechanism of IUGR individuals themselves. In the IUGR sucking lambs, the enhanced duodenal mitophagy was also found[37]. These results in this study directly or indirectly demonstrated that IUGR induced hepatic mitochondrial damage owing to mitochondrial OXPHOS disorders. As expected, RSV supplementation improved hepatic mitochondrial OXPHOS and mitophagy-related gene expression in IUGR piglets. The improvement of hepatic mitochondrial OXPHOS resulting from RSV supplementation was also observed in both suckling[4] and finishing[18] IUGR pigs. RSV has been reported to regulate mitophagy through the activation of key molecular targets such as AMP-activated protein kinase, the mechanistic target of rapamycin, deacetylases, and mitochondrial quality control pathways, which have been reviewed in detail by Liu et al.[38]. However, the role of mitophagy in the protective effects of RSV on hepatic damage in the IUGR weaned piglets still needs to be further investigated.
-
In summary, this finding presented that RSV supplementation could protect against liver injury in IUGR weaned piglets. The ability of RSV to enhance antioxidant defence, inhibit inflammation, and facilitate mitochondrial OXPHOS may converge in the restoration of hepatic homeostasis. This insight may help in the development of appropriate nutritional strategies to ameliorate hepatic damage in IUGR weaned piglets.
This research was funded by the National Natural Science Foundation of China (Grant No. 32202722), the Undergraduate Research Training (Integration of Science and Education) Project at Henan University of Technology of China (Grant No. KYXL2025200), and the Natural Science Foundation of Henan Province of China (Grant No. 242300420142).
-
All procedures were reviewed and preapproved by the Ethics Management Committee of Henan University of Technology, identification number: HAUT20230301, approval date: 2023.03.01. The research followed the 'Replacement, Reduction, and Refinement' principles to minimize harm to animals. This article provides details on the housing conditions, care, and pain management for the animals, ensuring that the impact on the animals was minimized during the experiment.
-
The authors confirm their contributions to the paper as follows: study conception and design: Cheng K; methodology: Cheng K, Yao J; formal analysis: Chang C; investigation: Cheng K, Yao J, Chang C, Han Y, Yu H; draft manuscript preparation: Cheng K; writing—review and editing: Song Z, Li Z, Zhang X, Bao H; supervision: Wang J, Zhao H; project administration: Zhang Y; funding acquisition: Cheng K, Huang J. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed in the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Composition and nutrient levels of the basal diet (air-dry basis).
- Supplementary Table S2 List of primers used in RT-qPCR.
- Copyright: © 2026 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cheng K, Yao J, Chang C, Song Z, Han Y, et al. 2026. Dietary resveratrol supplementation alleviates liver damage induced by intrauterine growth retardation in weaned piglets. Animal Advances 3: e007 doi: 10.48130/animadv-0025-0049
Dietary resveratrol supplementation alleviates liver damage induced by intrauterine growth retardation in weaned piglets
- Received: 19 October 2025
- Revised: 27 November 2025
- Accepted: 18 December 2025
- Published online: 10 February 2026
Abstract: Here, the effect of resveratrol (RSV) on hepatic redox status, inflammation, mitochondrial function, and mitophagy in weaned piglets with intrauterine growth retardation (IUGR) was investigated. A total of 12 normal birth weight (NBW, ~1.59 kg), and 24 IUGR ( ~0.94 kg) newborn piglets were obtained from 12 sows. At 26 d of age, all piglets were weaned and allocated into three groups for a 21-d trial. Treatments included: (1) NBW piglets fed a basal diet; (2) IUGR piglets fed a basal diet; (3) IUGR piglets fed a RSV-supplemented basal diet. Serum and liver samples were collected at the end of this trial. Compared with NBW, IUGR reduced absolute liver weight and increased serum aspartate aminotransferase (AST) activity (p < 0.05). RSV reversed the elevated serum AST activity in IUGR piglets (p < 0.05). RSV restored the reduced total superoxide dismutase activity, and increased interleukin 18 and interleukin 1 beta levels in the liver of IUGR piglets (p < 0.05). IUGR decreased adenosine triphosphate (ATP) and complex I concentrations, and increased BCL2/adenovirus E1B 19-kDa-interacting protein 3-like (BNIP3L) mRNA expression in the liver (p < 0.05). RSV increased (p < 0.05) ATP content and reduced (p = 0.086) BNIP3L transcriptional expression in the liver of IUGR piglets (p < 0.05). Results suggest that RSV alleviates liver damage in IUGR weaned piglets by improving redox status, inflammation, and mitochondrial function, providing a theoretical basis for future rational utilization of RSV in swine production.
-
Key words:
- Intrauterine growth retardation /
- Resveratrol /
- Liver damage /
- Redox status /
- Mitophagy














