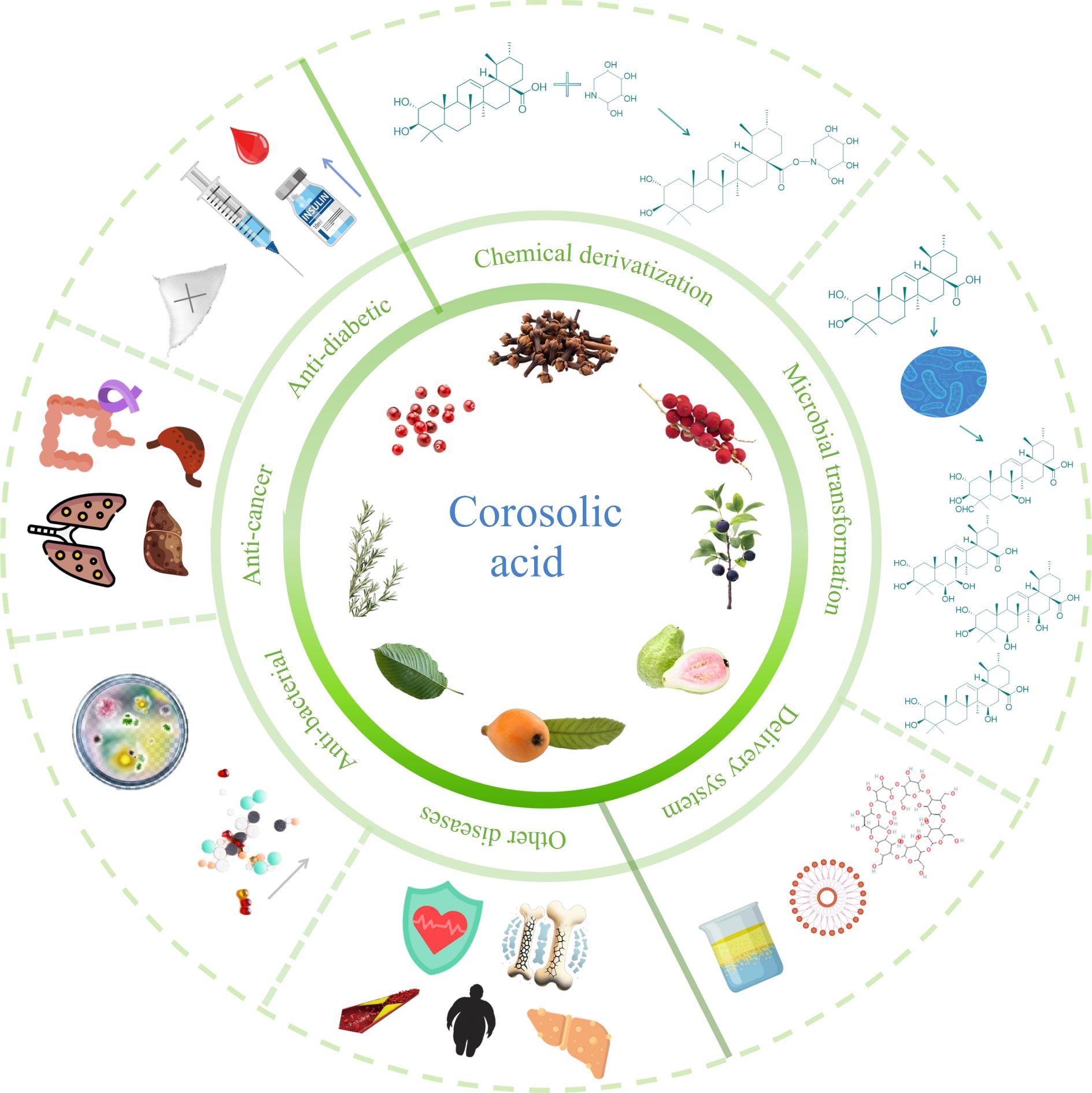
-
Corosolic acid, a ursane-type pentacyclic triterpenic acid, is a naturally occurring compound widely distributed in various plants, including banaba[1], loquat leaves[2], guava[3], rosemary[4], allspice, clove[5], cranberry[6], sweet osmanthus[7], and black cherry[8]. Early studies focused on determining its chemical structure and its biological function in plants. More recently, corosolic acid has garnered significant attention from the scientific community, particularly in the field of diabetes research, due to its widespread availability and diverse biological activities[9]. Corosolic acid and its structural analogs, including ursolic, oleanolic, maslinic, asiatic, glycyrrhetinic, madecassic, moronic, pomolic, boswellic, and betulinic acids, have been reported to exhibit various biological activities, such as anti-diabetic, anti-cancer, anti-inflammatory, and antimicrobial effects[9−11]. Among these, corosolic acid has demonstrated a stronger potential anti-diabetic effect compared to other pentacyclic triterpenic acids[12,13].
Many studies have highlighted the therapeutic potential of corosolic acid in various areas. Its anti-diabetic properties are primarily attributed to the inhibition of enzymes such as α-amylase[13], α-glucosidase[12], and diabetes-related non-receptor protein tyrosine phosphatases[14], as well as its ability to enhance insulin signaling pathways[15]. As for the application in the food field, corosolic acid has been used as a key ingredient in dietary supplements to support glucose metabolism and maintain healthy blood sugar levels[1].
With the increasing body of research on corosolic acid, its pharmacological effects beyond anti-diabetes have been increasingly revealed. In addition to its anti-diabetic properties, corosolic acid has demonstrated multiple other biological activities. Its anti-cancer effects have been reported through mechanisms such as inducing ferroptosis[16,17], promoting apoptosis in cancer cells[18,19], and inhibiting cancer cell migration and invasion[20]. Furthermore, corosolic acid exhibits anti-inflammatory effects by suppressing pro-inflammatory cytokines and key inflammatory pathways[21,22]. Additionally, reports indicate that corosolic acid acts as a biofilm inhibitor[23], demonstrating antibacterial properties and enhancing the bactericidal activity of antibiotics[24], thereby further expanding its application potential. These diverse bioactivities suggest that corosolic acid holds significant promise as both a dietary supplement and a pharmaceutical adjunct.
Corosolic acid, as a triterpenoid compound, is biosynthesized from 2,3-oxidosqualene, a common C30 acyclic precursor produced through the mevalonate pathway[25]. The molecular structure of corosolic acid consists of a side chain containing both an alcohol group and a carboxylic group, as well as a cyclic triterpenoid skeleton. Corosolic acid suffers from limited solubility in water and low bioavailability due to its lack of polar functional groups and its long lipophilic backbone, which restrict its practical applications in food and pharmaceutical fields[26]. To overcome these limitations, several studies have explored strategies to enhance its functionality and bioavailability, including chemical modifications[27], microbial transformations[28], and delivery systems such as liposomes[29], and nanoemulsion[30].
This review provides an overview of recent advances in the functional activities of corosolic acid and discusses emerging strategies aimed at enhancing its functional properties and therapeutic efficacy (Fig. 1). It aims to contribute to the growing body of knowledge on corosolic acid, highlighting its potential as a valuable bioactive compound and guiding future research into its applications in food and pharmaceuticals.
-
Corosolic acid initially attracted attention for its potential therapeutic effects on diabetes, earning it the nickname 'phyto-insulin' or 'botanical insulin'[31]. Its impact on glucose metabolism and insulin sensitivity has made it a compound of considerable interest in diabetes research. Corosolic acid has shown a promising impact in managing diabetes, particularly type 2 diabetes, through several mechanisms that enhance insulin sensitivity and regulate glucose metabolism[32]. One of its primary therapeutic actions is the inhibition of α-glucosidase activity, primarily governed by hydrophobic interactions, which delays glucose absorption and helps manage postprandial hyperglycemia[12,13,33,34] (Fig. 2). Corosolic acid inhibits both α-glucosidase and α-amylase in a non-competitive, reversible manner, with an IC50 value of 1.35 × 10−5 mol/L for α-glucosidase inhibition—less than half the value of the positive control acarbose, demonstrating a higher inhibitory effect on α-glucosidase[12,13]. Furthermore, when combined with myricetin, corosolic acid exhibits a synergistic effect on α-glucosidase inhibition, further supporting its role in glucose regulation[12].
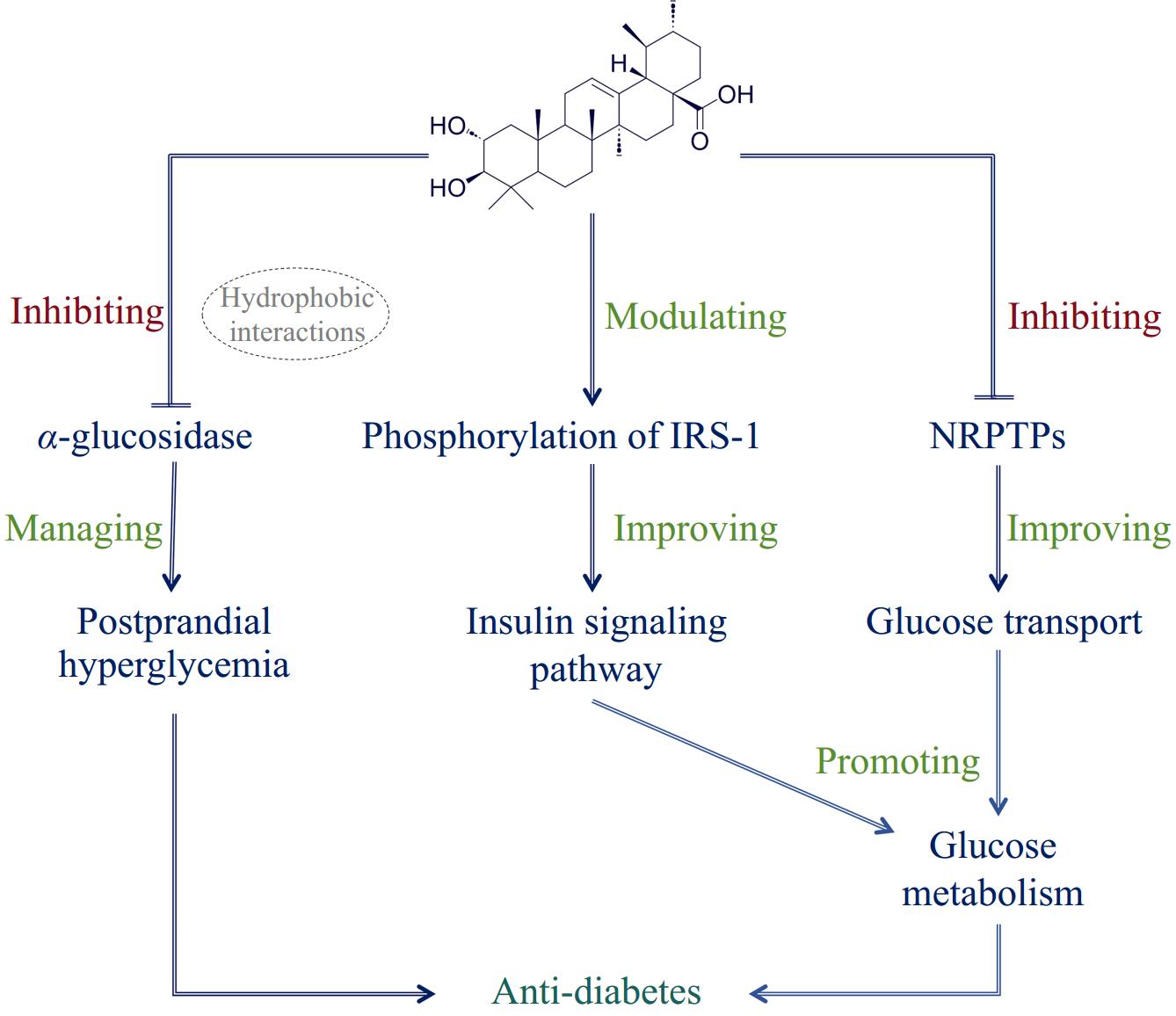
Figure 2.
Proposed mechanisms of corosolic acid in diabetes inhibition. IRS-1: Insulin Receptor Substrate-1; NRPTPs: Non-Receptor Protein Tyrosine Phosphatases.
In addition to its effects on glucose absorption, corosolic acid has been shown to enhance insulin signaling pathways. It improves insulin sensitivity in high-fat diet-induced diabetic mice by modulating the phosphorylation of Insulin Receptor Substrate-1 and its downstream target, Protein Kinase B[15,25]. This modulation enhances the insulin signaling cascade, contributing to improved glucose homeostasis. Corosolic acid also activates AMP-activated protein kinase, a key regulator of energy metabolism, which inhibits inflammation and reduces insulin resistance[15,35]. Furthermore, studies have demonstrated that corosolic acid reduces the phosphorylation of IκB kinase β and suppresses the expression of pro-inflammatory cytokines, alleviating adipose tissue inflammation—a key contributor to insulin resistance[15].
In vitro studies also highlight the role of corosolic acid in glucose uptake. It enhances glucose uptake in L6 myotubes by inhibiting several diabetes-related non-receptor protein tyrosine phosphatases and promoting the translocation of glucose transporter type 4 in CHO/hIR cells, suggesting its potential to improve cellular glucose transport[14,36], further contributing to the regulation of glucose metabolism. The anti-diabetic effects of corosolic acid extend to animal models of type 2 diabetes, such as the KK-Ay mouse, a model of insulin resistance and hyperglycemia[37]. In these mice, a single oral dose of corosolic acid (2 mg/kg body weight) significantly reduced blood glucose levels within 4 h, with sustained effects over 2 weeks[38]. Moreover, corosolic acid treatment lowered plasma insulin levels and improved glucose tolerance in the insulin tolerance test, indicating a reduction in insulin resistance. One study investigated the effects of corosolic acid in a randomized, double-blind, crossover trial involving 14 middle-aged, non-diabetic men with impaired fasting glucose tolerance. The results suggested that corosolic acid may enhance insulin action and improve glucose metabolism in prediabetic individuals[39].
Overall, the ability of corosolic acid to improve glucose metabolism and insulin sensitivity, without inducing anti-insulin antibodies, supports its potential as a therapeutic agent for managing type 2 diabetes.
Anti-cancer effect
-
Apart from its anti-diabetic properties, corosolic acid has demonstrated significant potential as an anti-cancer agent through various mechanisms, positioning it as a promising compound for cancer treatment, as highlighted by increasing studies on its anti-cancer activity[40,41]. Table 1 summarizes the proposed inhibitory mechanisms of corosolic acid against different types of cancer. One of its most notable anti-cancer properties is its ability to induce ferroptosis, a form of regulated cell death characterized by iron-dependent lipid peroxidation[42,43]. Experimental results have further confirmed the role of ferroptosis in the anti-cancer action of corosolic acid, as ferroptosis inhibitors can effectively rescue corosolic acid-induced lung cancer cell death, while corosolic acid itself suppresses epithelial-mesenchymal transition and induces ferroptosis independently of the inhibitors[16]. Corosolic acid has also been reported to sensitize liver cancer cells to ferroptosis[17] and reduce the proliferation and invasion of pancreatic cancer cells by inducing SAT1-mediated ferroptosis[2], marking it as a novel approach in cancer therapy. Additionally, corosolic acid has demonstrated notable cytotoxicity under hypoxic conditions, commonly found in tumors, suggesting its potential as an adjunctive treatment for cancer[41]. A concentration of 4 µM corosolic acid significantly inhibits the growth of A549 lung cancer cells[44].
Table 1. Proposed inhibitory mechanisms of corosolic acid against different types of cancer.
Cancer Proposed inhibitory mechanisms Ref. Lung cancer Inhibition of epithelial-mesenchymal transition, promoting ferroptosis [16,44] Liver cancer Promoting ferroptosis, downregulating the VEGFR2/Src/FAK/cdc42 axis, suppressing the CDK19/YAP/o-GlcNAcylation pathway, activating the PERK-eIF2α-ATF4 pathway [19,45,46] Gastric cancer Modulating the PI3K-AKT signaling pathway, influencing AMPK-mTOR signaling pathway, inhibiting HER2 gene expression [18,47,48] Colon cancer Promoting N-terminal phosphorylation of β-catenin, reducing COX-2 expression, activating caspase-3 [49−51] Pancreatic cancer Promoting ferroptosis, inhibiting the JAK2/STAT3 signaling pathway [2,52] Kidney cancer Reducing MMP2 expression and stimulates ERK1/2 phosphorylation, stimulating ERK1/2 phosphorylation [20,42] Breast cancer Reducing the phosphorylation levels of JAK2 and STAT3 proteins and activating apoptosis-related caspases [53] Bladder cancer Inhibit DNA synthesis, downregulating cell cycle regulators, triggering autophagy-related pathways [54] Ovarian cancer Inhibiting macrophage-epithelial interactions [55] Oral cancer Inhibits the expression of MMP1 [56] Bone cancer Disrupting mitochondrial membrane potential, increasing the Bax/Bcl-2 ratio, releasing cytochrome c, activating
caspases-8, -9, and -3[57] Effect on liver cancer
-
Corosolic acid can target key signaling pathways directly or exert anticancer effects on various cancers through synergistic interactions with other substances. In liver cancer, corosolic acid interacts with the ATP-binding pocket of VEGFR2 kinase, thereby inhibiting its activity and downregulating the VEGFR2/Src/FAK/cdc42 axis, which is essential for liver cancer cell migration and invasion[45]. Additionally, corosolic acid suppresses the CDK19/YAP/o-GlcNAcylation pathway, a mechanism critical for the proliferation of high-glucose cancer cells, thus inhibiting liver cancer cell growth[46]. Furthermore, corosolic acid activates the PERK-eIF2α-ATF4 pathway, inducing endoplasmic reticulum stress-mediated apoptosis in hepatocellular carcinoma cells[19]. In vitro studies have demonstrated that corosolic acid inhibits the proliferation of liver cancer cells, including Bel-7404 and HepG2, in a dose-dependent manner, resulting in G2/M cell cycle arrest and promoting cell death[58].
Effect on gastric cancer
-
Molecular docking studies indicate that corosolic acid interacts with key targets, including PIK3CA, PTGS2, AKT1, and CASP3, modulating the PI3K-AKT signaling pathway (hsa04151) to exert anticancer effects[48]. Additionally, corosolic acid enhances the sensitivity of gastric cancer cells to the chemotherapy drug 5-fluorouracil by activating AMP-activated protein kinase (AMPK)[47]. Through regulation of the AMPK-mTOR signaling pathway, corosolic acid inhibits mammalian target of rapamycin (mTOR) activity, thereby modulating cancer cell growth and apoptosis[59]. Furthermore, it has been shown to suppress HER2 gene expression, resulting in cell cycle arrest and apoptosis, which provides a theoretical basis for its application in treating HER2-positive gastric cancer[18]. In SNU-620 gastric cancer cells, corosolic acid further potentiates the anticancer efficacy of 5-fluorouracil by inhibiting mTOR signaling, underscoring its potential as an adjuvant therapy[60].
Effect on colon cancer
-
Corosolic acid demonstrates significant potential in combating colon cancer through multiple mechanisms. One study indicates that corosolic acid inhibits the proliferation of APC-mutant colon cancer cells by promoting N-terminal phosphorylation of β-catenin, leading to its subsequent proteasomal degradation[49]. Additionally, corosolic acid exhibits a strong binding affinity to COX-2 and effectively reduces its expression, highlighting its dual anti-inflammatory and anticancer properties[50]. In vitro studies further reveal that corosolic acid induces apoptosis in CT-26 cells by activating caspase-3. Furthermore, in vivo experiments using a mouse colon cancer model demonstrate that corosolic acid significantly reduces the final tumor volume, as well as the blood and lymphatic vessel density within the tumor[51]. Similarly, the molecular targets and signaling pathways of ursolic acid contribute to apoptosis in colon cancer cell lines by up-regulating caspase-3, -8, and -9 and activating the phosphoinositide 3-kinase and MAPK/extracellular signal-regulated kinase pathways[61].
Effect on other cancers
-
As research on corosolic acid progresses, its effects on various cancers are being increasingly explored. In pancreatic cancer, corosolic acid inhibits the JAK2/STAT3 signaling pathway, suppressing tumor progression[52]. For renal cell carcinoma, it reduces MMP2 expression and stimulates ERK1/2 phosphorylation in 786-O and Caki-1 cells, thereby preventing cancer cell migration and invasion through interactions with MMP2[20]. In breast cancer, corosolic acid induces apoptosis in MDA-MB-231 cells by downregulating JAK2 and STAT3 phosphorylation and activating apoptosis-related caspases[53]. Furthermore, corosolic acid has shown potential in reversing tumor-promoter-induced DNA methylation changes in mouse epidermal JB6 P+ cells[62].
In bladder cancer, corosolic acid suppresses TOP2A and LIG1 to inhibit DNA synthesis, downregulates cell cycle regulators, and triggers autophagy-related pathways involving proteins such as NBR1, TAXBP1, SQSTM1/P62, and UBB[54]. Additionally, corosolic acid enhances the efficacy of traditional chemotherapy agents like paclitaxel, cisplatin, and doxorubicin by suppressing macrophage-epithelial interactions that activate epithelial ovarian cancer cells[55]. In human oral squamous cell carcinoma, it significantly inhibits MMP1 expression and impairs cell migration and invasion, demonstrating synergistic effects when combined with siMMP1[56]. The isomer of corosolic acid, maslinic acid, has also been reported to effectively inhibit the viability of bladder cancer cell lines (T24, TCCSUP, 253J, and RT4), with IC50 values ranging from 27 to 71 µM[63].
Corosolic acid also induces G2/M cell cycle arrest in CaSki cervical cancer cells in a dose-dependent manner[64]. In MG-63 osteosarcoma cells, it disrupts mitochondrial membrane potential, increases the Bax/Bcl-2 ratio, releases cytochrome c, and activates caspases-8, -9, and -3, leading to apoptosis[57]. Collectively, these findings highlight the versatility of corosolic acid as an anticancer agent, targeting diverse mechanisms across various cancer types.
Protective effects against some diseases
-
Corosolic acid has been investigated for its broader biological activities beyond its well-established effects on diabetes and cancer, highlighting its potential as a multifunctional dietary supplement or therapeutic agent. One notable function is its ability to protect against diabetic nephropathy, a common complication of diabetes. Corosolic acid has been shown to inhibit the proliferation of glomerular mesangial cells via the p38 MAPK and NADPH-mediated ERK1/2 signaling pathways, offering protection against diabetic kidney damage[65].
Additionally, corosolic acid exerts significant cardioprotective effects, particularly in the context of ischemia-reperfusion injury[66]. It mitigates oxidative stress and preserves myocardial mitochondrial structure and function. Studies have demonstrated that corosolic acid treatment significantly reduces markers of myocardial injury, such as CK-MB and LDH levels, in rats subjected to isoproterenol-induced myocardial damage[67]. By decreasing lipid peroxidation and boosting endogenous antioxidant levels, corosolic acid improves cardiac function and protects against acute myocardial injury.
Corosolic acid also plays a role in protecting against heart failure and myocardial fibrosis. It attenuates myocardial infarction-induced cardiac fibrosis and dysfunction through modulation of inflammation and oxidative stress pathways, with AMPKα being a key regulator in this process[68]. Moreover, corosolic acid has been found to protect against doxorubicin-induced cardiac toxicity by activating AMPK-dependent mechanisms, including the activation of TFEB, which enhances autophagy and mitigates cardiac damage[69].
In vascular health, corosolic acid demonstrates potent vasorelaxant effects via activation of the NO/cGMP and H2S/ATP-sensitive potassium (KATP) channel pathways[70]. It also alleviates pulmonary arterial hypertension (PAH)-induced vascular remodeling by inhibiting the PDGF-PDGF receptor β-STAT3/NF-κB signaling axis, which is involved in PAH pathogenesis[71]. Its structural analog, ursolic acid, has also been reported to maintain cardiovascular health by regulating the expression of vascular injury factors, promoting vascular endothelial cell proliferation, and enhancing angiogenesis[72].
Beyond its cardiovascular effects, corosolic acid exhibits significant anti-osteoporotic properties. It inhibits the PI3K/AKT/mTOR signaling pathway, enhancing autophagy and preventing IL-1β-induced degradation of extracellular matrix in cartilage[73]. Additionally, corosolic acid reduces osteoclastogenesis and oxidative stress, offering protection against lipopolysaccharide-induced bone resorption[74]. The function was also found in asiatic acid, which can inhibit osteoclast formation through the RANKL-activated NF-κB or NFATc1 signaling pathways, thereby reducing bone resorption[75].
In metabolic disorders, corosolic acid controls lipid metabolism and reduces oxidative stress. It effectively inhibits pancreatic lipase and cholesterol esterase, enzymes responsible for the breakdown of triglycerides and cholesterol, thereby regulating lipid levels in the bloodstream[76]. Furthermore, corosolic acid improves hypertension, lipid metabolic abnormalities, oxidative stress, and inflammation in spontaneously hypertensive rats[77], suggesting its potential to prevent atherosclerosis-related diseases.
Corosolic acid has also been found to improve glucose metabolism by promoting glucose uptake in 3T3-L1 adipocytes and inhibiting adipocyte differentiation through downregulation of key adipogenic markers, such as PPAR-γ and C/EBP-α[78]. This effect positions corosolic acid as a promising candidate for managing obesity-related conditions and metabolic syndrome.
Finally, the rising prevalence of obesity and diabetes has significantly contributed to the widespread occurrence of non-alcoholic fatty liver disease, which often progresses to hepatocellular carcinoma[31]. Studies have shown that corosolic acid can markedly inhibit ethanol-induced cell apoptosis while increasing levels of tumor necrosis factor-α and reactive oxygen species, both in vitro and in vivo[31,79]. Furthermore, corosolic acid may protect the liver from ethanol-induced damage through the regulation of MAPK signaling pathways and activation of autophagy[79].
Antimicrobial properties
-
Corosolic acid has demonstrated significant potential as an antibacterial agent, with multiple studies highlighting its ability to inhibit the growth of various pathogenic bacteria, particularly those associated with hospital-acquired infections. Notably, corosolic acid exhibits stronger antimicrobial activity against Staphylococcus aureus (a Gram-positive bacterium) compared to Escherichia coli (a Gram-negative bacterium)[30], which is the same as other triterpenic acids, such as ursolic, asiatic oleanolic, and betulinic acids[80,81].
A key antimicrobial property of corosolic acid is its ability to prevent biofilm formation, a common feature of bacterial infections that contributes to antibiotic resistance[82]. Corosolic acid has been identified as an effective biofilm inhibitor[23,83]. It enhanced the sensitivity of biofilm-producing Pseudomonas aeruginosa to treatment with tobramycin, a widely used antibiotic for resistant infections[23], and improved the antibacterial activity of cefotaxime against S. aureus[83], suggesting its potential as an adjunct in combination therapy.
In addition to inhibiting biofilm formation and enhancing antibiotic efficacy, corosolic acid plays a synergistic antibacterial role in other ways. Corosolic acid analogs (oleanolic and ursolic acids) have been reported to impact cell morphology and promote autolysis of bacterial cells by influencing peptidoglycan metabolism[84]. Although less effective against Gram-negative bacteria compared to Gram-positive bacteria, it has been reported to enhance the bactericidal activity of antibiotics against E. coli. This effect is attributed to corosolic acid's inhibition of β-lactamase enzymes, such as KPC-2, which mediate antibiotic resistance in E. coli. By inhibiting β-lactamase activity, corosolic acid can restore E. coli's sensitivity to carbapenem antibiotics[24].
Additionally, corosolic acid has been identified as a promising natural product for the treatment of bee diseases, particularly for controlling infections caused by Melissococcus plutonius (European foulbrood) and Bacillus larvae (American foulbrood)[85]. These Gram-positive bacterial diseases can be controlled using corosolic acid, further demonstrating its broad-spectrum antimicrobial activity.
In summary, corosolic acid's ability to inhibit bacterial growth, prevent biofilm formation, and restore antibiotic efficacy positions it as a promising plant-derived natural product in the fight against antibiotic-resistant bacterial infections and the development of alternative antimicrobial therapies.
Regarding anti-fungal activities, corosolic acid has demonstrated varying potency depending on the fungal species. Studies have reported a minimum inhibitory concentration of 12.5 μg/mL against Ascosphaera apis, a fungal pathogen affecting honeybees[86]. However, at a concentration of 100 μg/mL, corosolic acid exhibited only a 22.16% inhibition rate against Sclerotinia sclerotiorum[87], indicating limited efficacy against this particular fungal strain.
Corosolic acid has also demonstrated notable antiviral activity. In vitro studies have shown that corosolic acid exhibits anti-respiratory syncytial virus and anti-herpes simplex virus type 1 activities, with IC50 values of 12.5 μM, comparable to the positive control ribavirin, which has an IC50 of 10 μM[88]. The antiviral mechanisms of corosolic acid may involve its interaction with the allosteric site of AMPK. By stimulating AMPK, corosolic acid can inhibit lipid biosynthesis, a process crucial for viral replication[89], further supporting the potential of corosolic acid as an antiviral agent.
-
The poor water solubility and low bioavailability of corosolic acid are primarily attributed to the rigid skeleton and hydrophobic nature of its pentacyclic triterpenic structure[90−93], which limits its application as both a dietary supplement and a therapeutic agent. To overcome these challenges, various strategies, including chemical derivatization, microbial transformation, and advanced delivery systems, have been explored. These approaches not only aim to improve the solubility and bioavailability of corosolic acid but also hold the potential to enhance its functional activity.
Chemical derivatization
-
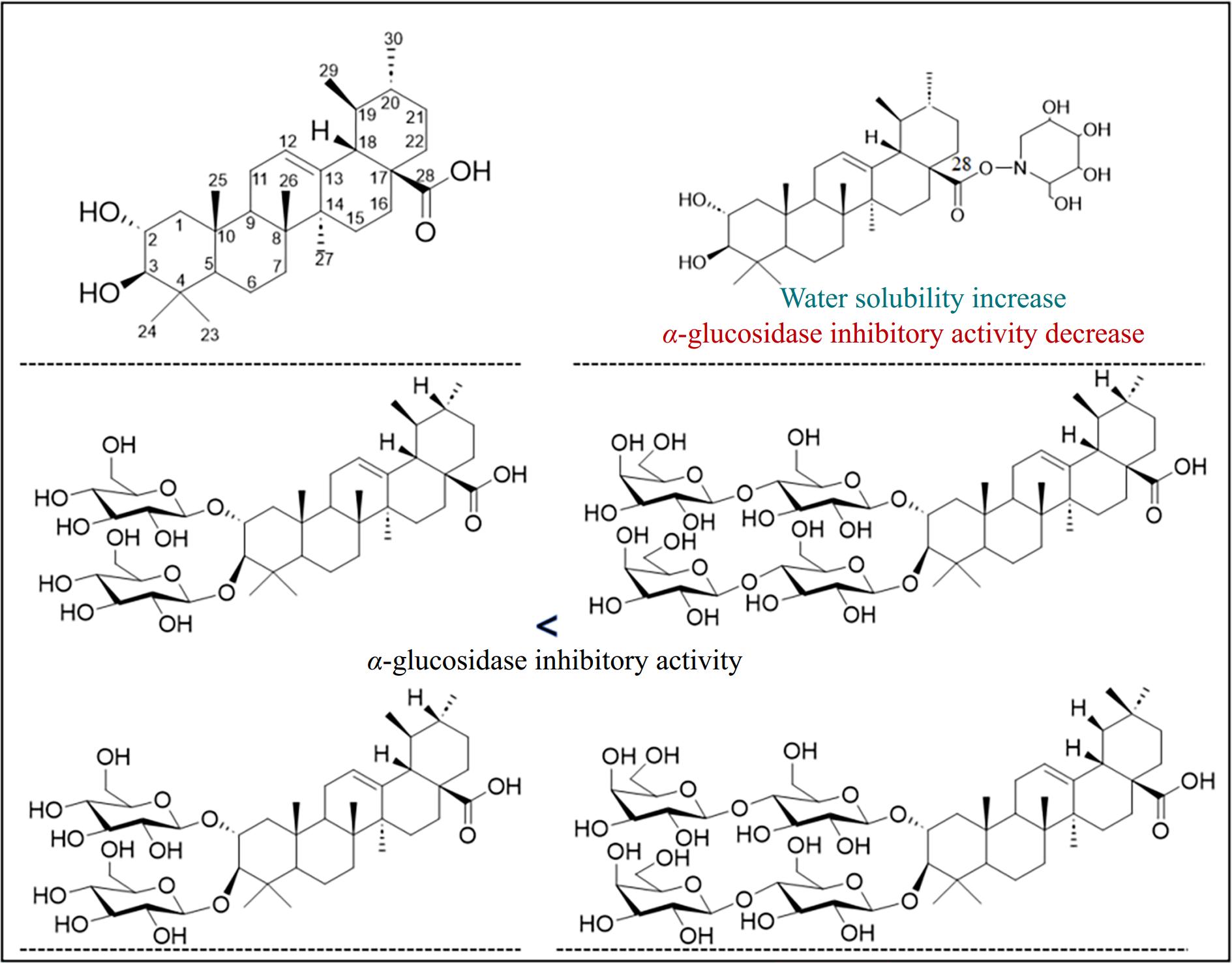
Chemical modification of natural compounds is a crucial strategy to improve solubility, bioavailability, and biological activity. This approach offers flexibility in designing compounds with improved specificity and efficacy for targeted applications[94]. The approach has been widely explored in other triterpenic acids, providing valuable insights into structure-activity relationships. By modifying the C-2, C-3, C-11, C-12, and C-28 positions of the oleanolic acid scaffold, its anti-diabetic, anticancer, and antiviral activities can be enhanced[90]. Similarly, the introduction of an amino group at the C-28 position of asiatic acid significantly enhances its anticancer activity against several cancer cell lines[95]. These findings suggest that specific functional group modifications could be a key factor in optimizing the biological activity of corosolic acid. By introducing functional groups or conjugating the compound with bioactive moieties, chemical derivatives can enhance its pharmacological effects while addressing its inherent limitations[96–97].
There was study that reported modifications at the C-28 position, such as the introduction of piperazine-L-amino acid complexes, have yielded derivatives with varied biological activities. However, the α-glucosidase inhibitory activity of these derivatives in DMSO and ethanol-water systems was lower than that of the parent compound[98] (Fig. 3). In contrast, other studies have shown that derivatives containing piperazine units exhibited superior activity compared to their precursors. Molecular docking simulations further revealed that the incorporation of piperazine enhanced hydrogen bonding interactions with α-glucosidase, thereby improving the biological activity of the derivatives[99]. The IC50 values of some piperazine derivatives was half of corosolic acid. Additionally, the carbon chain length of the linker between triterpenic acid and 1-DNJ plays a critical role in hypoglycemic activity, with shorter linkers exhibiting stronger inhibition of α-glucosidase than longer ones.
Quinoline derivatives of corosolic acid have also demonstrated promising antitumor properties. For example, 7-aminoquinoline, 5-aminoquinoline, and 8-isoquinoline derivatives exhibited high cytotoxicity, strong tumor cell selectivity, and the ability to overcome drug resistance[100]. The derivatization could decrease the drug resistance ratio more than twice. Glycosylation of corosolic acid with mono- and disaccharides improves its water solubility, with disaccharides showing superior solubility and α-glucosidase inhibitory activity compared to monosaccharides. However, their inhibitory activity (ranging from IC50 = 428 μM to no inhibition) was still much lower than that of the parent compound (71 μM)[101].
Furthermore, hybrid derivatives of corosolic acid have shown significant effects on tumor cell cycle progression at doses much lower than those required for the natural triterpenic precursors. Cationic derivatives, such as TPP+ and F16-triterpenoid conjugates, outperformed their parent compounds in inducing reactive oxygen species (ROS) and were more effective at reducing mitochondrial membrane potential in isolated rat liver mitochondria, highlighting the therapeutic potential of chemical derivatization to enhance the pharmacological properties of corosolic acid[27].
Microbial transformation
-
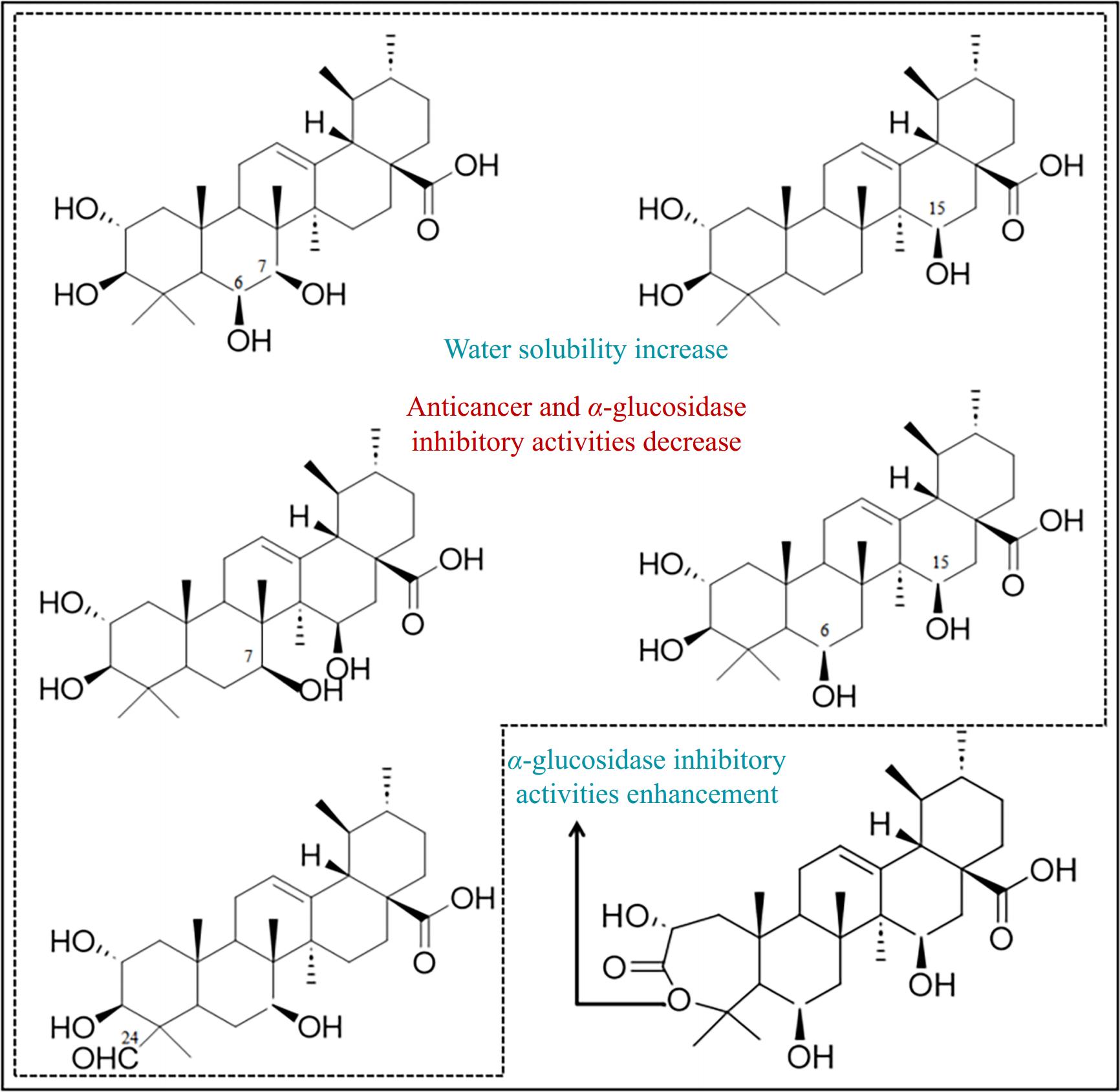
The second method, microbial transformation, is an attractive approach for expanding the structural diversity of triterpenoids by enabling unique modifications such as methylation, epoxidation, regioselective hydroxylation, and methyl migration[102–103].
Studies involving Fusarium equiseti and F. solani demonstrated selective oxidation of corosolic acid at the C-6, C-7, C-15, and C-21 positions, resulting in polar metabolites[104] (Fig. 4). Although these modifications enhanced the water solubility of corosolic acid, hydroxylation at these positions significantly reduced its anticancer and α-glucosidase inhibitory activities. Similarly, metabolism by Cunninghamella echinulata and C. blakesleeana generated new hydroxylated and oxidized metabolites, which also exhibited diminished α-glucosidase inhibition[28]. The study indicated that hydroxylation at the C-6 position and oxidation at the C-24 site of corosolic acid negatively impacted its α-glucosidase activity.
In contrast, another study showed that while C-15, C-21, and C-30 hydroxylation and C-21 carbonylation of corosolic acid reduced its α-glucosidase inhibitory activity, lactonization within the A-ring could enhance this activity[28]. Most microbial derivatives exhibited reduced toxicity toward cancer cells, likely due to decreased substrate toxicity resulting from microbial metabolism[105].
Based on these studies, microbial transformation can improve the bioavailability of corosolic acid, but it generally decreases its α-glucosidase inhibitory activity. However, analyzing the effects of these structural changes on the compound's function may offer valuable insights into optimizing the modification of corosolic acid for therapeutic applications[106].
Delivery system
-
Advancements in delivery systems have introduced novel strategies to enhance the solubility, stability, and bioavailability of triterpenic acids, including corosolic acid[107−109]. The supramolecular properties of corosolic acid allow it to molecular self-assembly without structural modifications supporting subsequent application[110–111]. For example, it self-assembles into vesicles or supramolecular gels in aqueous organic solvents[112], demonstrating potential for applications such as drug encapsulation, controlled release, and fluorescence labeling. Additionally, corosolic acid can spontaneously form inclusion complexes with hydroxypropyl-β-cyclodextrin, significantly improving its water solubility and enhancing its bioavailability[113].
Nanoemulsions of corosolic acid further enhance its functionality by reducing hydrophobicity, thus improving its antibacterial activity against Gram-positive bacteria[30]. Corosolic acid structural analogs, oleanolic acid, and ursolic acid, have also been confirmed as stabilizers for the preparation of Pickering emulsions[114,115]. This makes corosolic acid-based emulsions promising for applications in food, cosmetics, and pharmaceuticals. Cholesterol-free lipid nanoparticles derived from corosolic acid exhibit superior tumor cell uptake and endosomal membrane fusion capabilities, enabling more efficient cytoplasmic delivery of mRNA and siRNA[116].
In anticancer applications, corosolic acid-based liposomes demonstrate several advantages over conventional cholesterol-based liposomes. For instance, doxorubicin-loaded corosolic acid-based liposomes enhance membrane fusion, and cellular uptake, and inhibit STAT3 activation and macrophage recruitment in the tumor microenvironment[29]. Similarly, corosolic acid-loaded liposomes containing paclitaxel significantly improve its anticancer efficacy by overcoming tumor biological barriers, enhancing immunogenic cell death, and achieving satisfactory therapeutic outcomes[117]. These studies highlight the potential of corosolic acid-based delivery systems in improving solubility, stability, antibacterial, and anticancer activities. Such systems offer promising alternatives to traditional drug carriers, opening avenues for wide-ranging applications in medicine, food, and cosmetics.
-
This review offers a comprehensive and current analysis of the functional activities and biological activity enhancement strategies for corosolic acid. It's demonstrated therapeutic potential spans anti-diabetic, anti-cancer, anti-inflammatory, and antibacterial properties, with notable activities including enzyme inhibition (e.g., α-amylase and α-glucosidase), regulation of insulin signaling pathways, induction of ferroptosis, promotion of apoptosis, and suppression of pro-inflammatory cytokines and biofilm formation. These attributes position corosolic acid as a promising candidate for applications in dietary supplements and pharmaceuticals. However, its poor water solubility and limited bioavailability remain significant barriers to broader utilization. Strategies such as chemical modifications, microbial transformations, and advanced delivery systems (e.g., liposomes and nanoemulsions) have shown promise in overcoming these challenges, enhancing therapeutic efficacy, and broadening functionality. Advanced delivery systems, in particular, demonstrate significant potential to simultaneously improve solubility, stability, and biological activities. These advancements underscore the importance of optimizing corosolic acid's pharmacological profile for practical applications in food and medicine.
Future research should prioritize refining these enhancement strategies, elucidating the molecular mechanisms underlying its diverse bioactivities, and ensuring the long-term safety and efficacy of corosolic acid. Addressing these challenges will pave the way for corosolic acid to emerge as a highly valuable bioactive compound with broad applications in health and wellness.
This research is funded by the National Key Research and Development Program of China (No. 2023YFD2201300), and the Key Research and Development Program of Zhejiang Province (2023C02042), China.
-
The authors confirm contribution to the paper as follows: formal analysis, writing - original draft: Shi B; investigation: Shi B, Sun H; supervision: Jia Q, Luo Z; validation: Mao Y; conceptualization; funding acquisition: Luo Z; writing - review and editing: Sun Z, Zhang H, Luo Z. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Shi B, Sun H, Sun Z, Jia Q, Zhang H, et al. 2025. Biological activities and potential functional optimization strategies of corosolic acid: a review. Food Innovation and Advances 4(2): 228−237 doi: 10.48130/fia-0025-0023
Biological activities and potential functional optimization strategies of corosolic acid: a review
- Received: 09 January 2025
- Revised: 27 February 2025
- Accepted: 24 March 2025
- Published online: 25 June 2025
Abstract: Corosolic acid, a naturally occurring pentacyclic triterpenic acid, is widely recognized for its broad spectrum of biological activities, particularly its anti-diabetic properties, making it a popular ingredient in dietary supplements for regulating blood sugar levels. Beyond its anti-diabetic effects, recent studies have revealed its therapeutic potential in areas such as anti-cancer, anti-inflammatory, and antibacterial activities. However, its clinical application is hindered by poor water solubility and low bioavailability due to its molecular structure. This review systematically examines the pharmacological activities of corosolic acid, emphasizing its mechanisms of action in disease intervention. Emerging strategies to overcome its inherent limitations, including chemical modifications, microbial transformations, and advanced delivery systems, are also discussed. Notably, some chemical derivatives exhibit α-glucosidase inhibition with IC50 values half that of corosolic acid. Microbial transformations have been shown to enhance its bioavailability while reducing cancer cell toxicity. Additionally, corosolic acid-based delivery systems have demonstrated significant improvements in solubility, stability, and biological activity. By consolidating current insights into its functional properties and biological activity enhancement methods, this review aims to emphasize the practical application values in food and medicine and the future development of corosolic acid as a versatile bioactive compound.