-
Hyperbaric oxygen therapy is essential for newborns with low birth weight to support their lung development. However, it may lead to retinopathy of prematurity (ROP), resulting in ischemia-driven retinal neovascularization and even permanent vision loss[1−4]. Conventional treatments, such as laser therapy[5−7], anti-vascular endothelial growth factor (VEGF) therapy[8−11], and vitreous surgery[12−14], typically intervene in the later stages of the disease, when neovascularization has already formed. Therefore, elucidating the impact of early elevated oxygen concentration on retinal vasculature may offer novel insights into the early diagnosis and treatment of ROP, and significantly enhance our understanding of the pathogenesis of neovascular eye diseases.
The oxygen-induced retinopathy (OIR) mouse model mimics the pathological process of ROP, and it also serves as a valuable tool for investigating the pathogenesis of retinal neovascularization in conditions such as retinal vein occlusion and proliferative diabetic retinopathy. In this model, mouse pups and their nursing dam are exposed to a high-oxygen environment for a specified period, followed by a return to normal air, which sequentially leads to delayed angiogenesis, regression of established superficial retinal vessels, and ischemia-driven retinal neovascularization[15−17]. Currently, most studies focus on the later stages of abnormal neovascularization[18−21], while the earlier process of superficial vascular regression remains poorly understood. Previous studies have attributed hyperoxia-induced vascular regression to oxidative stress-induced damage and endothelial cell apoptosis[22−28]. Besides, the expression levels of several angiogenic cytokines, including VEGF-A and pigment epithelium-derived factor (PEDF), are also involved in this process and are significantly correlated with the extent of avascular zones induced by high oxygen[22,29−32]. However, the precise mechanisms underlying this pathophysiological process have yet to be fully elucidated. Recently, the concept of the neurovascular unit has been introduced, highlighting the importance of cell-to-cell interactions[33−35]. This implies that the relationship between blood vessels and the surrounding cells could be a novel and pivotal focus for exploring the mechanisms underlying retinal neovascular diseases.
Microglia, the resident immune cells of the retina, originate from the yolk sac progenitor cells. In addition to their roles in retinal neuron development, synaptic pruning, and immune surveillance of the extracellular environment, microglia are intimately involved in the formation and maintenance of retinal vasculature[36−38]. During early retinal vascular development, microglia migrate to the neuroretina, where they promote endothelial cell proliferation and migration, and contribute to the branching and anastomosis of the vascular network[39,40]. Moreover, microglia are also implicated in pathological retinal vascularization. It is well-established that microglia become highly activated, transitioning from a resting 'ramified' state to an activated 'amoeboid' state, and become enriched around pathological retinal neovessels by postnatal day 17 (P17) in the OIR model[41]. Numerous studies have further demonstrated that microglia may be regulated through multiple pathways, thereby engaging in and facilitating pathological angiogenesis in the OIR model via mechanisms such as metabolic alterations, exosome secretion, and necrotic apoptosis[42−47]. However, it remains unclear whether microglia are involved in the early retinal vascular regression process in the OIR model and how they are involved.
PLX5622 is a highly selective, blood-brain barrier-permeable small molecule inhibitor that specifically targets CSF1R, a factor crucial for microglial survival, differentiation, and proliferation. PLX5622 enables the rapid and specific pharmacological depletion of microglia and has been widely used in recent years to treat central nervous system diseases targeting microglia and in mechanistic studies[48,49]. Oral administration of PLX5622 could increase neural circuit connectivity and activity in the adult mouse cortex[50] and improve postoperative cognitive decline in rodents by reducing hippocampal inflammatory mediators and abrogating recruitment of CCR2+ leukocytes[51]. Additionally, PLX5622 efficiently alleviated neural and vascular damage in the retinas of diabetic mouse models[52]. When administered during the proliferation phase in the OIR mouse model, PLX5622 reduces pathological neovascularization at P17[53]. However, its effects on early retinal vascular regression in the OIR model have yet to be explored.
In this study, we employed the OIR mouse model to elucidate the spatiotemporal characteristics of early retinal vascular regression under high oxygen conditions. Concurrently, we revealed the migration and activation of microglia during this pathological process. Partial depletion of microglia using PLX5622 inhibited the process of retinal vascular regression. Furthermore, we employed RNA sequencing, bioinformatics analysis, and molecular biology experiments to explore the underlying molecular mechanisms. These findings shed light on the role of microglia in high oxygen-induced retinal vascular regression, providing novel insights for the diagnosis and treatment of related diseases.
-
Adult (male and female) and newborn C57BL/6J mice were obtained from the Ophthalmic Animal Laboratory, Zhongshan Ophthalmic Center, Sun Yat-sen University (Guangzhou, China). All the animal experiments were approved by the Institution Animal Care and Use Committee of Zhongshan Ophthalmic Center (Permit No.: SYXK (YUE) 2018-0189) and were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were kept on a 12-h light/dark cycle under controlled temperature and humidity conditions.
OIR mouse model
-
To establish a reliable OIR mouse model, 7-day-old C57BL/6J mouse pups and their lactating mothers were kept in a chamber with an oxygen concentration of 75% ± 2% for five days before being transferred to normoxia (OIR group). Extra nursing dams were prepared in case of fatal pulmonary edema. Body weights were monitored, and the overweight or underweight pups were excluded to minimize the influence of metabolic health. Age-matched pups kept in normoxia served as normal controls (NOX group). Both groups of mice were sacrificed at designated time points, and their eyeballs were extracted for further experimentation.
PLX5622 treatment
-
To deplete microglia in the retinas of the mouse model, PLX5622 (MCE, Monmouth Junction, NJ, USA) was administered to neonatal mice at a dose of 50 mg/kg daily via intraperitoneal injection, from P4 to P7. The dissolution and dilution of PLX5622 were performed following the manufacturer's instructions. Pups injected intraperitoneally with the vehicle, consisting of DMSO (Sigma Aldrich), ethoxylated hydrogenated castor oil (MCE), and saline at the same time points, served as vehicle controls.
Immunofluorescence
-
The eyes were enucleated and fixed in 4% paraformaldehyde, followed by the removal of the cornea and lens. For retinal flat mounts, the retinal pigment epithelium and sclera tissue were carefully dissected away, and the cup-shaped retinal tissues were radially cut into four-leaf clover shapes. For retinal cryosections, the cup-shaped ocular tissue was embedded in optimal cutting temperature (OCT) compound and sectioned into 10 μm thick slices. Both whole mounts and cryosections were blocked in a solution containing Triton X-100 (Sigma Aldrich) and 5% BSA (Biofroxx, Einhausen, Germany), followed by sequential incubation with primary antibodies (1:400), secondary antibodies (1:1000), and staining with DAPI (1 μg/mL; Sigma-Aldrich). Finally, an antifade mounting medium was applied for slide mounting. All antibodies used in this study are listed in Supplementary Table S1. Panoramic images of both retinal whole mounts and sections were acquired using the TissueFAXS Q+ system (TissueGnostics, Vienna, Austria), while detailed observations were performed with a confocal microscope (Zeiss, Oberkochen, Germany).
The area of avascular zones in the flat-mounted retina was identified and quantified using Fiji (National Institutes of Health, Bethesda, MD, USA)[54]. The length and area of vessels were measured with AngioTool (University of Warwick, UK)[55]. Four 40X fields of view corresponding to avascular zones were selected in each retina for the morphological analysis of microglia. The axon statistics of microglia, a robust indicator of activation level, were also carried out by the skeleton plug-in in Fiji, which was consistent with a previous study[38].
The number of microglia in retinal sections was quantified to determine their longitudinal migration pattern under high oxygen stimulation. Retinal sections through the optic nerve were evenly divided into three regions based on their distance from the optic nerve: the central zone (C), the mid-peripheral zone (MP), and the peripheral zone (P). Vertically, the sections were stratified according to retinal anatomical layers: the nerve fiber and ganglion cell layer (NFL + GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), and the outer nuclear layer (ONL). The microglia count in each zone was recorded, and the results were summarized.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay and CD31 staining
-
Retinal cryosections were subjected to Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay, followed by CD31 staining. TUNEL assay (Elabscience, Wuhan, China) was performed following the manufacturer's protocols. CD31 immunofluorescent staining was carried out using previously described methods. Nuclei were counterstained with DAPI for 10 min. Images were acquired using a confocal microscope.
Western blotting
-
OIR and NOX mice were sacrificed after 16 h of high-oxygen stimulation, and retinas were harvested for protein extraction using RIPA buffer (Beyotime, Shanghai, China) supplemented with PMSF (Beyotime). Protein concentrations were determined using a BCA assay kit (Beyotime). The protein samples were then subjected to electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked and incubated with the appropriate primary antibody (1:1000) overnight at 4 °C, followed by incubation with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (1:10000) at room temperature for 2 h. Protein bands were visualized using ECL high-sensitivity chemiluminescence reagent (Millipore, Burlington, MA, USA), and their intensities were quantified using Fiji software. The antibodies utilized in this experiment are detailed in Supplementary Table S1.
Quantitative real-time PCR
-
Retinal tissue collection from OIR and NOX mice was performed after 16 h of high-oxygen stimulation, following the protocol described above. Total RNA was extracted from the retinas using a Trizol reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Approximately 500 ng of RNA was used for reverse transcription with HiScript Q RT SuperMix (Vazyme, Nanjing, China). Quantitative real-time PCR was performed to measure the expression level of target genes using ChamQ SYBR Color qPCR Master Mix (Vazyme, Nanjing, China), with β-actin serving as the internal reference. The primer sequences used in this experiment are listed in Supplementary Table S2.
Transcriptome sequencing and bioinformatics analysis
-
The two groups of mice (OIR and OIR + PLX5622) were sacrificed, and total RNA was extracted from the retinal tissues using a Trizol reagent kit according to the manufacturer's protocol. After assessing RNA quality, mRNA was enriched and reverse-transcribed to synthesize cDNA. PCR amplification was then performed to construct a gene library after purifying and repairing the double ends of the cDNA. The library construction and sequencing were conducted using Illumina Novaseq6000 (Gene Denovo Biotechnology Co, Guangzhou, China). Differentially expressed genes (DEGs) were identified using the 'DESeq2' package. DEGs with a fold change ≥ 1.5 and p value < 0.05 were chosen for further analysis. Pathway enrichment analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Statistical analysis
-
All quantitative data were presented as mean ± standard deviation (SD). Intergroup differences between mean values were analyzed using a two-tailed, unpaired Student's t-test for comparisons between two groups and ordinary one-way ANOVA for comparisons involving more than two groups. Statistical significance was set at p < 0.05, and is denoted as * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. The results reported in this study were derived from three independent experimental replicates. Data analysis and statistical computations were performed using GraphPad Prism v9.4 (GraphPad Software, La Jolla, CA, USA).
-
The regression of retinal vascular induced by hyperoxic stimulation was the initial pathological process in the OIR model. To investigate the characteristic of the retinal vascular regression, P7 pups were exposed to 75% oxygen for varying durations: 2 h (P7 + 2 h), 6 h (P7 + 6 h), 12 h (P7 + 12 h), 1 d (P8), 3 d (P10), 5 d (P12), and 5 d followed by 1 d of recovery in normal air (P13), respectively. The results revealed that although the high-oxygen stimulus persisted for 5 d, segmental vascular collapse in the central region of the OIR retina occurred at a very early stage. Specifically, the central avascular zone first enlarged after 12 h of excessive oxygen stimulation. The area ratio of the central avascular region to the whole retina had reached 39.87% after 1 d, approximated to its maximum of 41.57%, which was observed after 5 d of hyperoxic stimulus at P12 when the OIR mice were moved out from the oxygen chamber (Fig. 1b). In summary, the retinal vessels regressed after short-term high-oxygen stimulation.
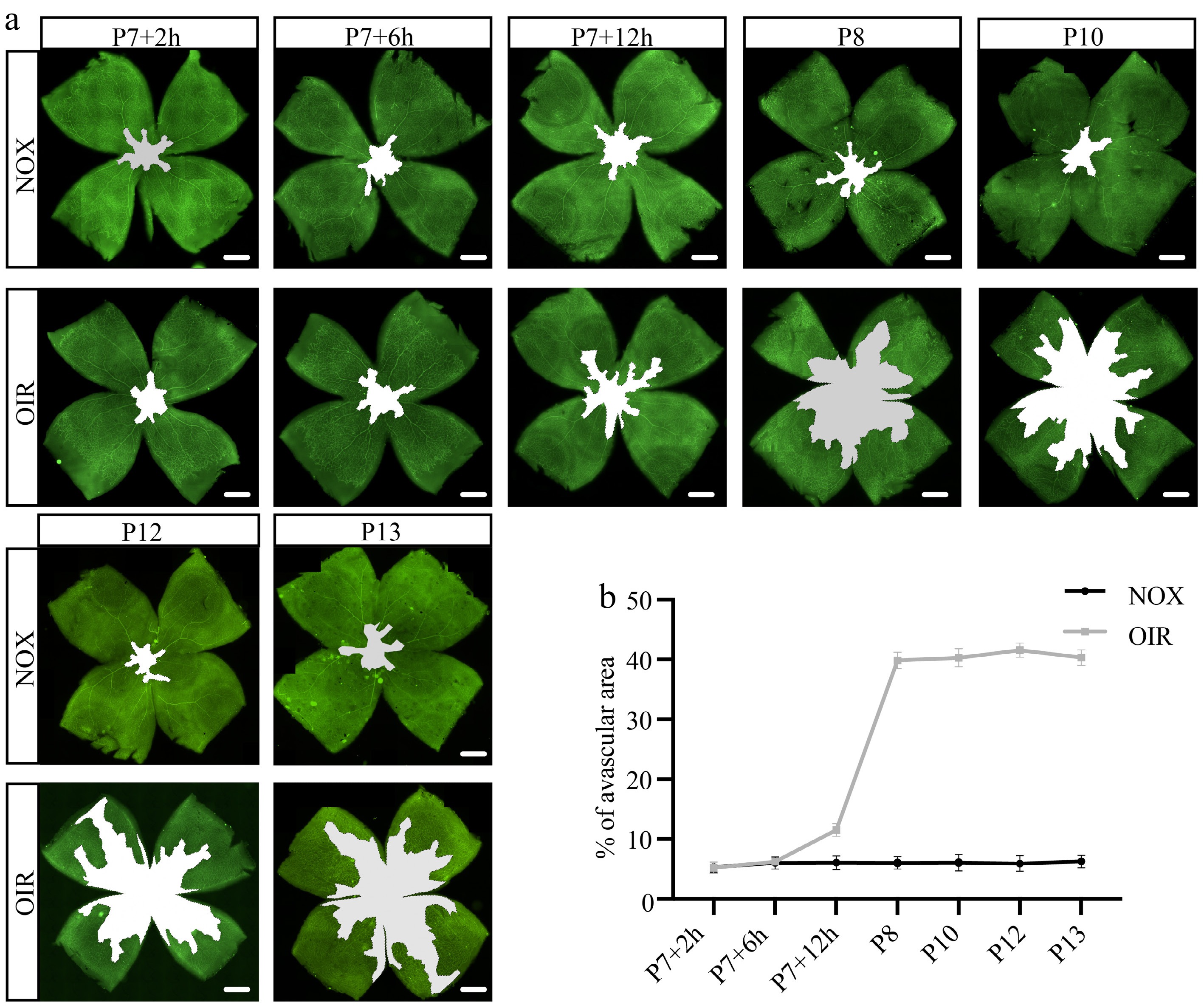
Figure 1.
Characteristics of retinal vascular regression under hyperoxic stimulation. (a) Representative panoramic images of retinal flat mounts stained with CD31 (green) at various time points, the white regions indicate avascular areas. Scale bar: 500 μm. (b) Proportion of avascular area in whole retina at different time points under hyperoxic stimulation. N = 5/group.
Migration and activation of retinal microglia after hyperoxic stimulation
-
To verify whether microglia are involved in the process of vascular rupture and subsequent clearance of vascular fragments, we performed ionized calcium-binding adapter molecule 1 (Iba1) immunofluorescent staining of retinal cryosections. We selected P7 + 16 h, the time point at which peak retinal vascular regression occurs, to conduct further observations. The retinal cryosections were divided into distinct regions, and the number of Iba1+ microglia was counted separately in each region (Fig. 2a). Our findings proved that although hyperoxia stimulation does not influence the total number of retinal microglia, their distribution has been changed. Interestingly, we found more Iba1+ microglia in NFL + GCL of the central retina under high-oxygen conditions compared to normal air. In contrast, the number of Iba1+ microglia decreased both in INL of the central retina and NFL + GCL of the peripheral retina (Fig. 2b). These results indicated the migration of microglia towards the innermost layer of the central retina, which corresponds spatially and temporally to the breakdown of retinal vessels under high oxygen stimulation.
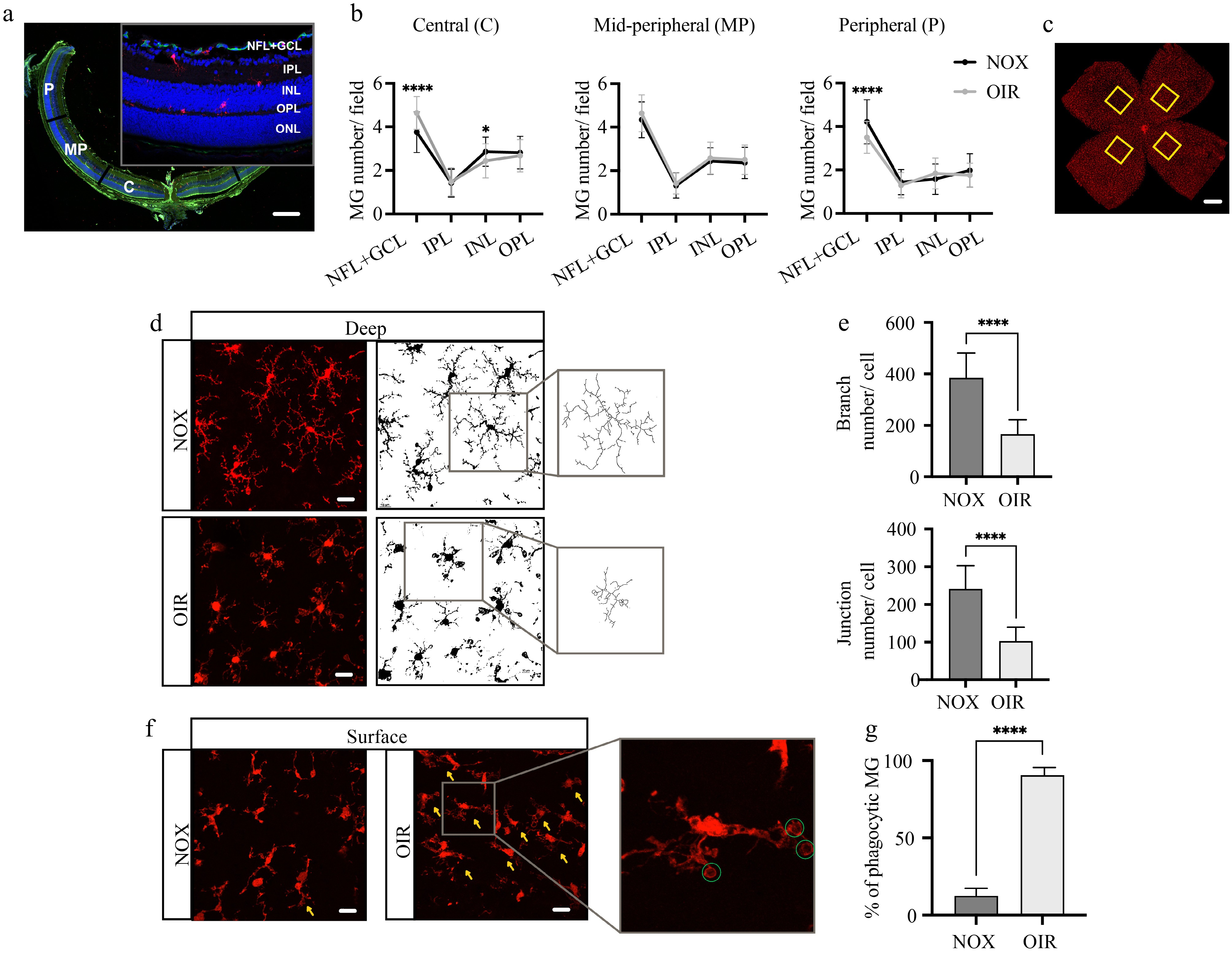
Figure 2.
Migration and activation of retinal microglia following short-term hyperoxic stimulation. (a) Representative image of retinal sections stained with CD31 (green), Iba1 (red), and DAPI (blue), illustrating the retinal regions and layers used for cell counting. Scale bar: 200 μm. (b) Quantification of microglial numbers in different regions at P7 + 16 h. N = 5/group. (c) Representative image of retinal flat mounts stained with Iba1 (red), the yellow boxes indicate areas selected for high-magnification imaging and subsequent cellular morphology analysis. Scale bar: 500 μm. (d) The morphological features of deep Iba1+ (red) microglia at P7 + 16 h, along with representative binary and skeletonized images of the microglia generated using Fiji software. Scale bar: 20 μm. (e) Quantification of the average branch and junction numbers per cell. N = 5/group. (f) The morphological features of superficial Iba1+ (red) microglia at P7 + 16 h, the yellow arrowheads indicate phagocytic morphology with 'ball-and-chain' structures, and the green circles highlight the phagocytic cups. Scale bar: 20 μm. (g) The percentage of phagocytic microglia per field. N = 5/group.
Since the morphology of microglia can reflect its function and activated state, we observed the morphological characteristic of microglia within retinal flat mount stained with Iba1 at P7 + 16 h (Fig. 2c). Overall, the superficial microglia (mainly located at NFL + GCL) exhibited a relatively activated morphology compared to the deep microglia (corresponding to the deep vascular network located at OPL) under both normoxia and hyperoxia. We analyzed the microglial skeleton in the deep retina using Fiji. The results showed that compared with the NOX group, microglia in the OIR group have fewer branches and endpoints (Fig. 2d, e). It suggested that microglia are relatively activated in a hyperoxic environment. A previous study reported that the 'ball-and-chain' structure of the 'phagocytic cup' is related to the phagocytic phenotype of microglia[56]. Our findings indicated that, compared to the NOX group, hyperoxic stimulation caused more microglia to exhibit the 'ball-chain' phagocytic phenotype in the superficial layer of the retina (Fig. 2f, g). Together, these results suggested that retinal microglia migrate and activate under high-oxygen stimulation.
Retinal microglia phagocytize vascular endothelial cells under hyperoxia
-
Double staining of Iba1 and CD31 was performed at P7 + 16 h to investigate the relationship between microglia and hyperoxia-induced vascular breakdown. Intriguingly, numerous microglia containing CD31+ endothelial cell debris were observed in the OIR retina, while no such phenomenon had been observed in the retina of the NOX group (Fig. 3a, b). This finding provided direct and compelling evidence that microglia are involved in hyperoxia-induced vascular breakdown through phagocytosis.
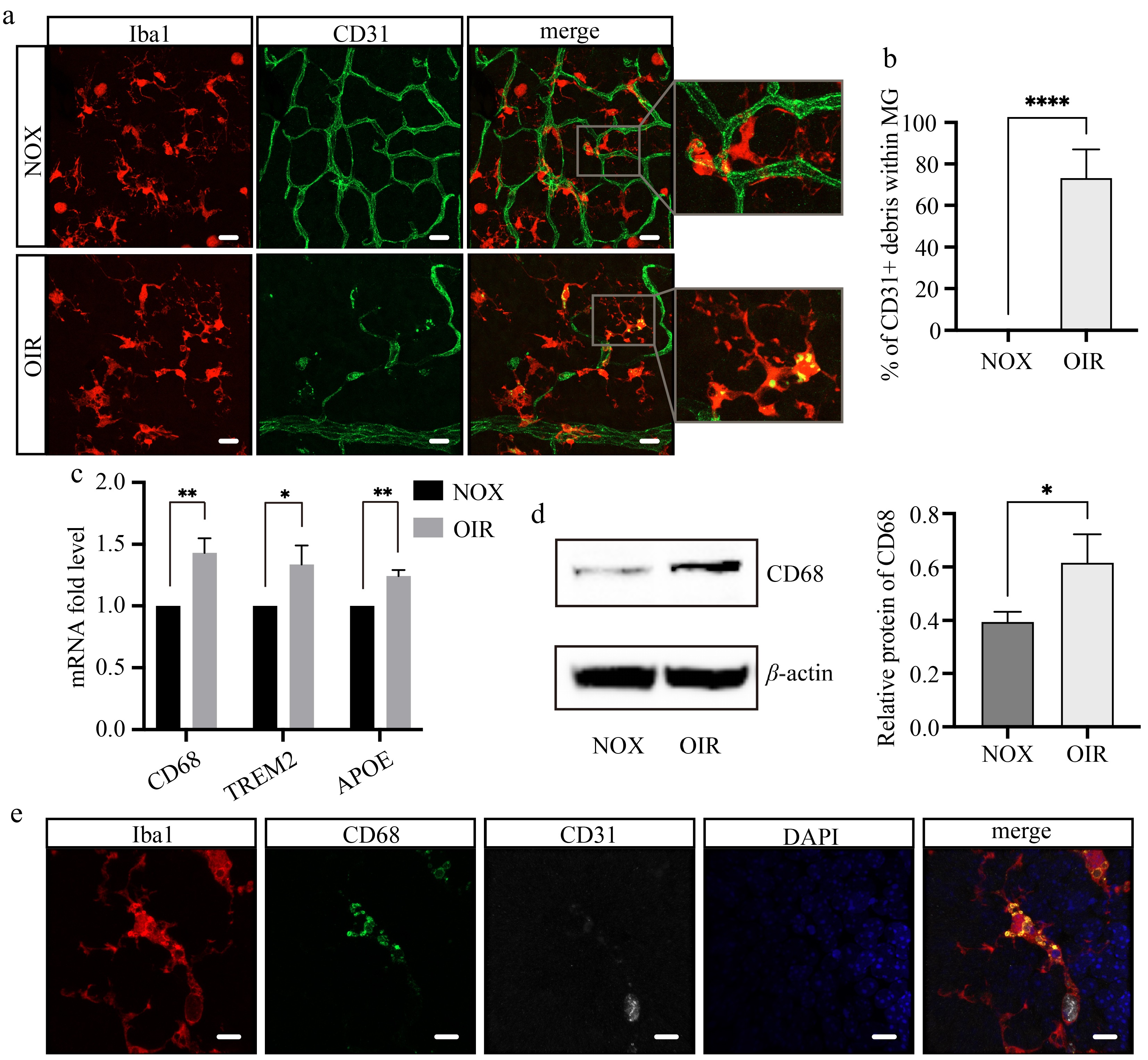
Figure 3.
Retinal microglia phagocytize vascular endothelial cells under hyperoxia. (a) Confocal images of retinal flat mounts stained with Iba1 (red) and CD31 (green) at P7 + 16 h. Scale bar: 20 μm. (b) The proportion of microglia containing CD31+ debris per field. N = 5/group. (c) The mRNA expression levels of genes related to phagocytosis. N = 3/group. (d) The protein expression and relative level of CD68. N = 3/group. (e) Multiple staining of Iba1 (red), CD31 (white), CD68 (green), and DAPI (blue) on the wholemount retina of OIR mice. Scale bar: 10 μm.
To explore the phagocytic capacity of retinal microglia, we extracted RNA from the whole retina and conducted quantitative real-time PCR assays for several phagocytosis-associated genes[57,58]. The results showed a significant increase in the mRNA expression of CD68, triggering receptor expressed on myeloid cells 2 (TREM2), and apolipoprotein E (APOE) following hyperoxic stimulation (Fig. 3c). CD68 is a specific marker for activated phagocytic microglia and macrophages. Immunofluorescence staining exhibited that CD68 expression in both the NOX and OIR groups was localized exclusively within Iba1+ microglia in the retina (Supplementary Fig. S1), indicating that CD68 level directly reflects the phagocytic capacity of microglia. We further confirmed that high-oxygen treatment upregulates CD68 expression at the protein level using Western blot analysis (Fig. 3d). In addition, multiple immunofluorescent staining of Iba1, CD31, and CD68 revealed that Iba1+ microglia, which is co-localized with CD31+ endothelial cell fragments, also express high levels of CD68 (Fig. 3e), verifying the phagocytic phenotype of these cells. Our findings thus demonstrated the phagocytic activity of microglia in response to hyperoxia stimulation.
Partial depletion of microglia inhibits early retinal vascular breakdown induced by hyperoxia
-
We depleted microglia with PLX5622, a highly selective and blood-retinal barrier permeable CSF1R inhibitor, to further elucidate the vital role of microglia. The medication treatments were administered from P4 to P7, the pups with their nursing dams were put into the oxygen chamber after the last injection, and euthanized at different time points for further experiments (Fig. 4a). At P7 + 16 h, over 60% of microglia were efficiently depleted (Fig. 4b, c) without affecting their survival or nutritional status (Supplementary Fig. S2). CD31 immunofluorescence staining of retinal flat mount revealed that the vessels of the PLX5622-treated group remained almost intact whereas the central retinal vessels in the vehicle group experienced segmental collapse after 16 h of hyperoxic stimulation (Fig. 4d). Statistically significant differences were observed between the two groups regarding their respective vessel area, vessel length, and the number of junctions (Fig. 4e). However, with prolonged high-oxygen exposure for 5 d, vascular breakdown in the central retina still occurred in the PLX5622-treated group, but the avascular zone in the central retina was smaller compared to the vehicle group, while continuous administration of PLX5622 from P4 to P11 did not affect the avascular area at P12 compared to the vehicle group (Fig. 4f, g). These results collectively suggested that retinal vascular changes induced by oxygen fluctuations might be regulated by multiple mechanisms, with microglia performing different functions at different stages.
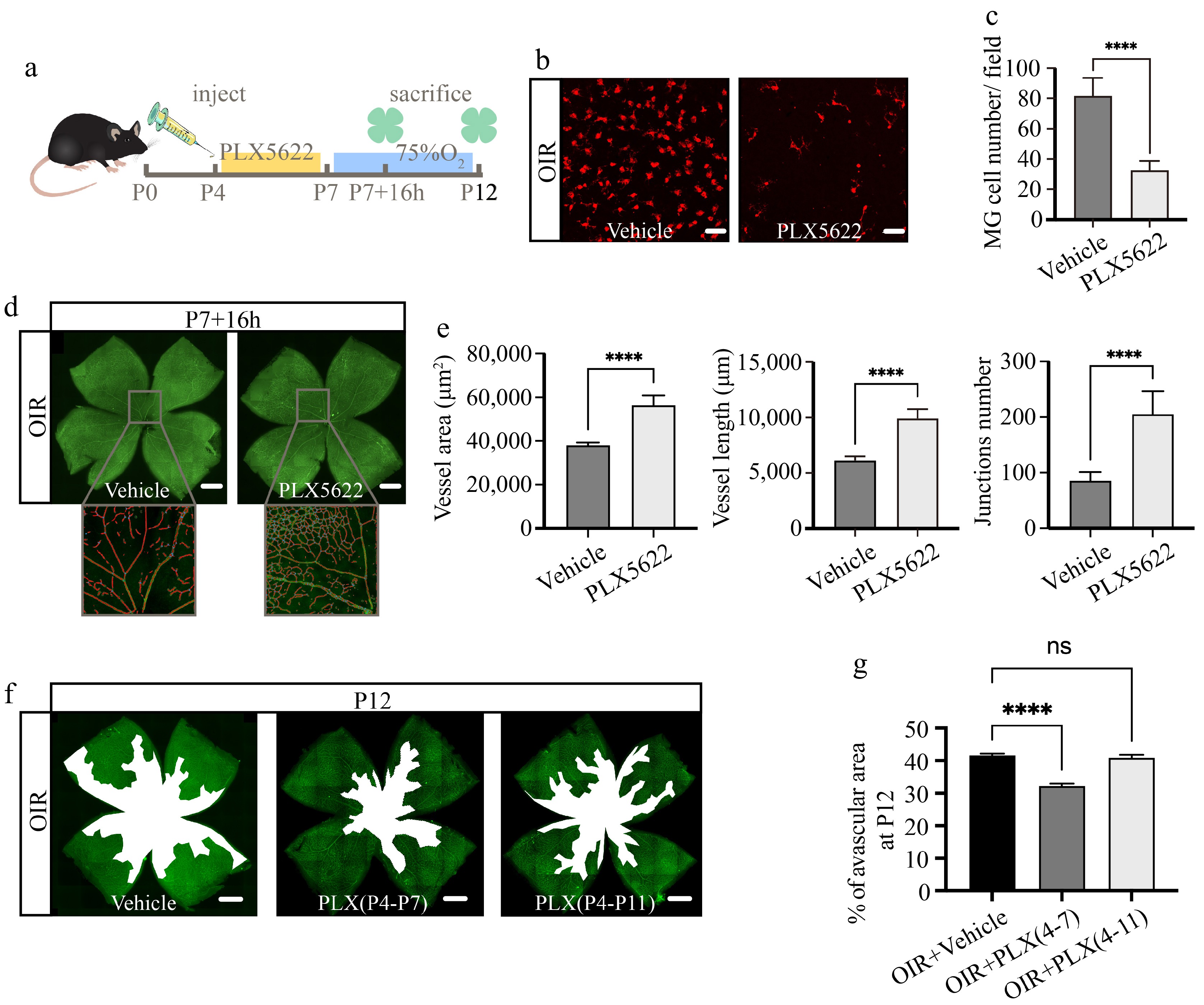
Figure 4.
Partial microglial depletion inhibits early retinal vascular breakdown induced by hyperoxia. (a) Schematic diagram and timeline of microglial depletion and subsequent observation. (b) Immunofluorescence staining of Iba1 (red) on retinal flat mounts at P7 + 16 h. Scale bar: 50 μm. (c) Quantification of Iba1+ microglial numbers per field. N = 5/group. (d) Immunofluorescence staining of CD31(green) on retinal flat mounts, with magnified vascular structure diagrams at P7 + 16 h. Scale bar: 500 μm. (e) Quantification of vessel area, vessel length, and junction numbers per field. N = 5/group. (f) Panoramic images of retinal flat mounts stained with CD31 (green), the white regions indicate avascular areas. Scale bar: 500 μm. (g) Proportion of avascular area in whole retina at P12. N = 5/group.
Microglial depletion alleviates endothelial cell apoptosis under high-oxygen conditions
-
To investigate whether the phagocytic role of microglia initiatively contributes to the breakdown of retinal vessels or passively participates in the clearance of fragmented vascular tissue after breakdown, we conducted apoptosis detection in retinal cryosections of mice with partial microglial depletion at P7 + 16 h. The results revealed that, compared to the normoxic control group, the hyperoxic group exhibited more TUNEL+ apoptotic cells. However, the total number of apoptotic cells was comparable with or without PLX5622 treatment (Fig. 5a, b). Although microglial depletion did not alter the total number of apoptotic cells, TUNEL and CD31 co-staining revealed a significant reduction in the proportion of apoptotic vascular endothelial cells with microglial depletion under hyperoxia conditions (Fig. 5c). It strongly confirmed the initiative involvement of microglia in the process of retinal vascular breakdown.
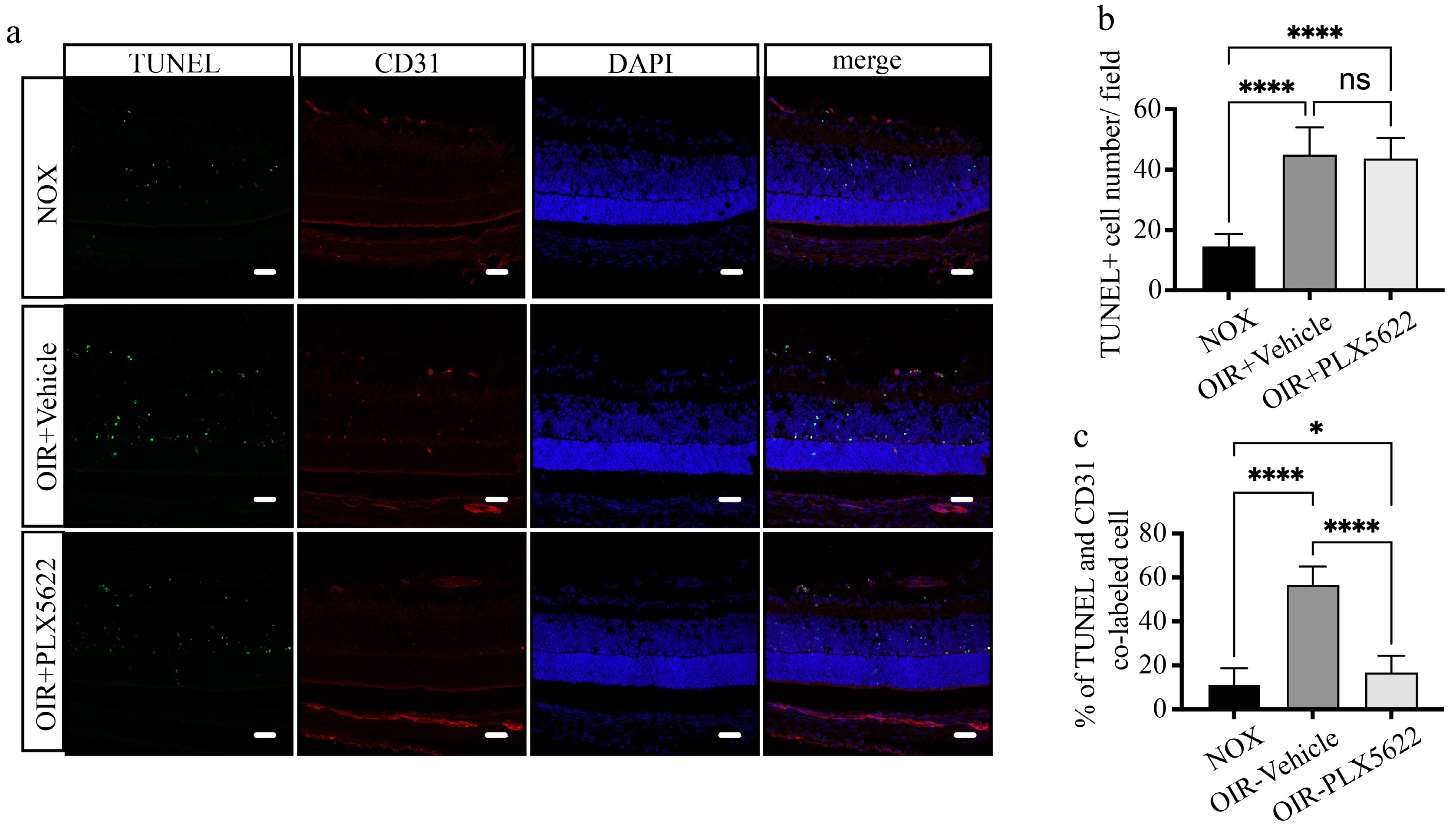
Figure 5.
Microglial depletion alleviates early endothelial cell apoptosis under high-oxygen conditions. (a) Representative images of retinal cryosections stained with TUNEL (green), CD31 (red), and DAPI (blue) at P7 + 16 h. Scale bar: 30 μm. (b) Quantification of TUNEL+ apoptotic cell numbers per field. N = 5/group. (c) The proportion of apoptotic endothelial cells co-labeled with TUNEL and CD31. N = 5/group.
The complement pathway regulates hyperoxia-induced microglial phagocytosis
-
Transcriptome sequencing was performed at P7 + 16 h to investigate the mechanisms underlying microglial phagocytosis under hyperoxic conditions. A total of 139 upregulated and 435 downregulated DEGs were identified between the PLX5622 + OIR group and the OIR + Vehicle group (Fig. 6a, b). KEGG pathway enrichment analysis of the 435 downregulated DEGs revealed that five of the top 10 enriched pathways were related to vascular inflammation and angiogenesis (Fig. 6c), indicating that microglial depletion indeed impacts the retinal vasculature in the OIR model. Additionally, the complement pathway was highly enriched among the downregulated DEGs. Representative genes from the complement pathway were selected for quantitative real-time PCR validation, and their expression was further examined in the NOX group. The results confirmed that complement pathway-related genes (C1qa, C1qb, C1qc, C3ar1, C4b) were upregulated under hyperoxic conditions and downregulated following microglial depletion (Fig. 6d). Notably, immunofluorescence triple staining revealed that C3ar1 was detected in phagocytic microglia containing CD31+ endothelial cell debris in the retinal flat mounts of the hyperoxia-treated group, suggesting its critical role as a regulatory factor (Fig. 6e). These findings demonstrated that the complement pathway regulates microglial phagocytosis under hyperoxic conditions.
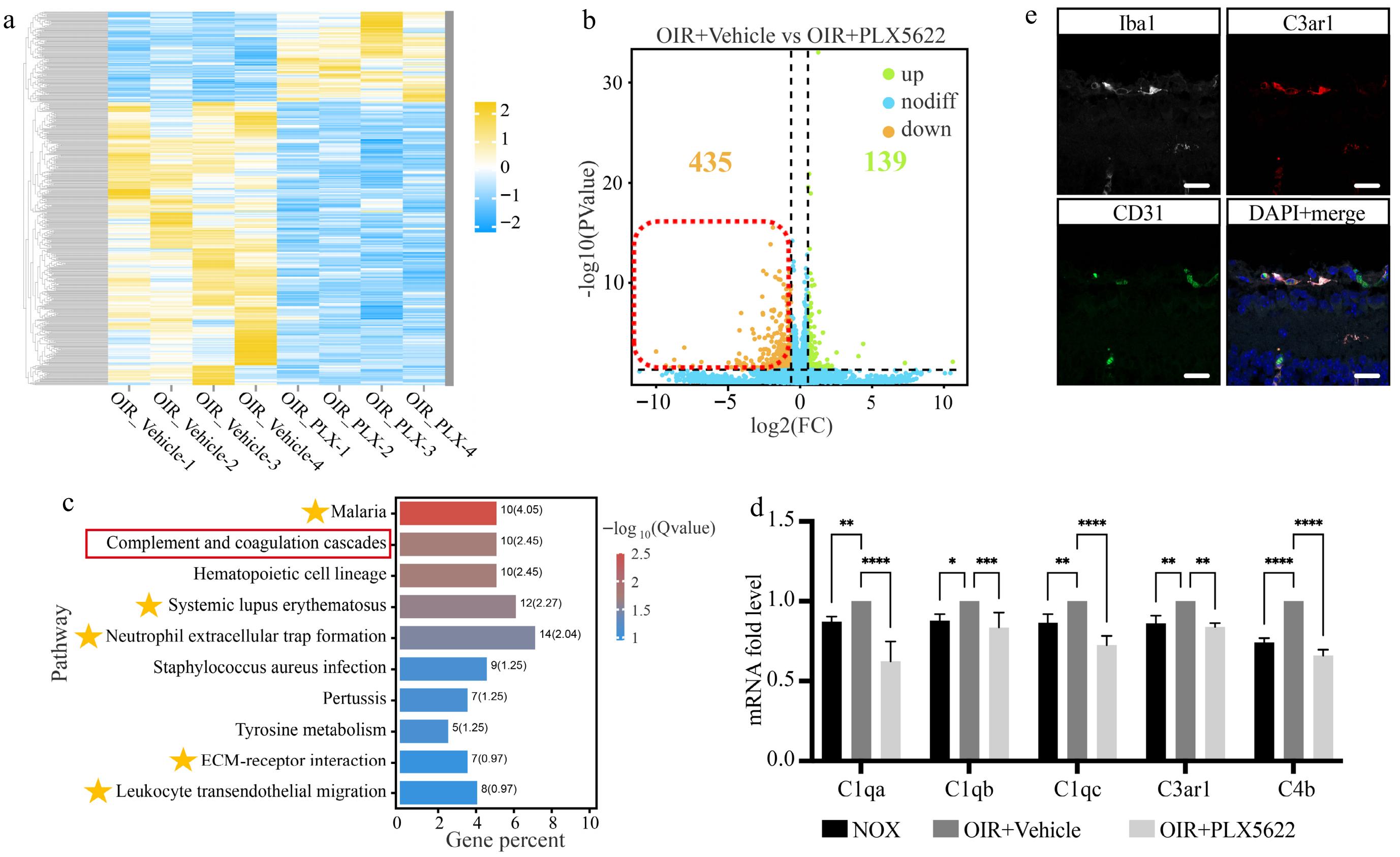
Figure 6.
Hyperoxia-induced microglial phagocytosis is regulated by the complement pathway. (a) - (b) Heatmap and volcano plots of DEG distribution between the OIR + Vehicle and OIR + PLX5622 groups. (c) Top 10 pathways identified by KEGG enrichment analysis of downregulated DEGs. The yellow stars indicate pathways related to vascular inflammation and angiogenesis. (d) The mRNA expression level of the key genes in the complement pathway. N = 3/group. (e) Multiple staining of Iba1 (white), CD31 (green), C3ar1 (red), and DAPI (blue) on the retinal cryosection of OIR + Vehicle mice. Scale bar: 10 μm.
-
Vascular regression is the initial pathological change of the retina under fluctuations in environmental oxygen concentration. Previous studies often considered the early appearance of avascular areas and subsequent neovascularization as two independent events, with a primary focus on the pathological processes and mechanisms of the latter, while paying less attention to the former. CD31, also known as platelet-endothelial cell adhesion molecule, is a member of the immunoglobulin superfamily. Although it is widely expressed on the surface of various cell types, including endothelial cells, platelets, neutrophils, monocytes, and certain types of T cells, it is still commonly used as a constitutive marker for vascular endothelial cells. In this study, CD31 was employed as a marker to achieve clear labeling of retinal blood vessels. In this study, we first observed that although the hyperoxia condition persists for 5 d, both retinal vessel regression and subsequent debris clearance manifest expeditiously, initiating after 12 h of hyperoxia stimulation and concluding within the following 12 h (Fig 1). In-depth research into hyperoxia-induced retinal vascular regression can enhance the overall understanding of neovascular diseases and provide new insights for their treatments.
Microglia, serving as immune surveillance cells resident in the central nervous system and retina, possess phagocytic abilities similar to macrophages and function as custodians for maintaining homeostasis within the organism. The phagocytic activity of microglia represents a double-edged sword. In the central nervous system, for instance, microglia mitigate the progression of neurodegenerative diseases, such as Alzheimer's, by engaging in the phagocytosis of aberrantly accumulated metabolic waste[59,60]. Conversely, excessive phagocytosis of neuronal axons by microglia exacerbates functional impairments within the nervous system[61]. Our observations revealed that microglia exhibit directed migration towards avascular regions, displaying a notably activated cellular morphology and high phagocytic activity, with CD31+ endothelial cell fragments within it (Figs 2 & 3a). These findings indicate that microglia might play a detrimental role in maintaining normal retinal vasculature during the early stages. Targeting microglia for early intervention may offer a new therapeutic strategy for oxygen-induced retinal neovascular diseases, such as ROP.
Numerous studies have reported that microglia become activated in various pathological processes. Pharmacological depletion of microglia has been widely used in mechanistic studies of various diseases and is considered a promising therapeutic strategy. In central nervous system diseases, the depletion of microglia can inhibit the pathological manifestations related to psychosocial stress and behavioral deficits associated with depression[62], alleviate neurodegeneration in Parkinson's disease[63], and delay the onset of autoimmune encephalomyelitis[64]. Additionally, microglial depletion within the eye could effectively mitigate retinal ganglion cell loss following optic nerve injury, and reduce vascular and neuronal damage in the retinas of diabetic mice[65,66] However, microglial depletion is not always beneficial to the organism. In mice with acute infectious encephalomyelitis, microglial depletion exacerbates demyelination severity and impairs myelin regeneration[67]. Similar adverse effects have also been observed in studies related to neonatal hydrocephalus[68]. It highlights the complexity of microglial functions, as they may play markedly different roles in various pathophysiological processes. Furthermore, depleting microglia at different time points within the same disease model can lead to varying outcomes[69,70], underscoring the critical importance of timing in microglia-targeted therapies. In our study, we depleted over 60% of microglia using PLX5622 before entering the oxygen chamber, effectively inhibited early vascular breakdown induced by hyperoxia at P7 + 16 h, and decreased the central avascular area at P12. Additionally, partial depletion of microglia significantly inhibited endothelial cell apoptosis (Figs 4d−g, 5). Our findings demonstrate for the first time that microglia are involved in the regression of normal vasculature before promoting hypoxia-induced retinal neovascularization. Early depletion of microglia before the proliferative phase may represent a novel strategy for early intervention in diseases such as ROP.
Moreover, our study also demonstrated that microglial phagocytosis under hyperoxia conditions may be modulated by the complement pathway. As an integral component of the innate immune system, the complement system can be activated by pathogenic microorganisms, initiating a series of cascade reactions to neutralize toxins, facilitate inflammation, and enhance opsonization, thereby regulating the immunological milieu. The regulation of microglia by the complement pathway and its underlying mechanisms have garnered significant attention from researchers. Within the central nervous system, microglia can mediate age-associated cognitive deficits through complement-dependent synaptic phagocytosis[71]. Besides, excessive synaptic phagocytosis by microglia, which is modulated by the complement signaling pathway, has been implicated in the etiology of depression and Huntington's disease[72,73]. The complement C3a receptor 1 (C3ar1) is a typical G protein-coupled receptor, and microglia with elevated C3ar1 expression have been observed to accumulate around amyloid plaques in murine models of Alzheimer's disease[74]. Similarly, in a mouse model of retinal detachment, heightened C3ar1 expression has been detected within retinal microglia, which is hypothesized to be associated with neuroinflammation and photoreceptor cell apoptosis[75]. Our study confirmed that C3ar1 expression is upregulated under hyperoxia stimulation, and the immunofluorescence staining further verified that its regulatory target is located on microglia (Fig. 6c, d). This suggested that C3ar1 might be the key target in regulating microglial phagocytic activity under hyperoxia conditions.
In conclusion, this study elucidated the mechanism of early retinal vessel regression under high-oxygen stimulation and further highlighted the critical role of microglia in this process. These findings might enhance comprehension regarding the pathogenesis of ROP and other oxygen-induced neovascular diseases, providing novel strategies for its early interventions.
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos 82271093 and 82070972). The authors thank the staff of the Public Experimental Platform and Laboratory Animal Center of Zhongshan Ophthalmology Center for assistance in the experiments.
-
All the animal experiments were approved by the Institution Animal Care and Use Committee of Zhongshan Ophthalmic Center (Permit No.: SYXK (YUE) 2018-0189) and were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research as well. The research followed the 'Replacement, Reduction, and Refinement' principles to minimize harm to animals.
-
The authors confirm contribution to the paper as follows: study conception and design: Xu Z, Ma Y, Zhuang J, Chen Z, Li T, Zhuang J; data collection: Xu Z, Li J, Liu Y, Lin Z, Liu B, Zhu Z, Wei X; analysis and interpretation of results: Xu Z, Jiang L, Tuxun R, Chen Z, Tsai CL; draft manuscript preparation: Xu Z, Zhuang J, Li T. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated and analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest. Dr. Zhuoting Zhu is the Editorial Board member of Visual Neuroscience who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research group.
- Supplementary Table S1 Antibodies for Immunofluorescence and Western blot.
- Supplementary Table S2 Primers used in this study.
- Supplementary Fig. S1 Multiple staining of Iba1 (red), CD31 (white), and CD68 (green) on the whole mount retina of OIR mice. Scale bar: 20 μm.
- Supplementary Fig. S2 Comparison of body weight among different treatment groups. N = 8/group.
- Copyright: © 2025 by the author(s). Published byMaximum Academic Press, Fayetteville, GA. This articleis an open access article distributed under Creative CommonsAttribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xu Z, Ma Y, Li J, Zhuang J, Liu Y, et al. 2025. Microglial phagocytosis involves hyperoxia-induced vessel regression in the neonatal retina. Visual Neuroscience 42: e004 doi: 10.48130/vns-0025-0004
Microglial phagocytosis involves hyperoxia-induced vessel regression in the neonatal retina
- Received: 11 February 2025
- Revised: 20 February 2025
- Accepted: 26 February 2025
- Published online: 27 March 2025
Abstract: Oxygen concentration fluctuations are fundamental contributors to retinopathy of prematurity and other retinal neovascular diseases. However, the mechanism of retinal vascular regression under high oxygen stimulation remains unclear. In this study, we employed an oxygen-induced retinopathy mouse model to investigate the role of microglia in hyperoxia-induced vessel regression. Retinal vascular regression characteristics, microglial morphology, and localization were observed using immunofluorescence staining. Expression levels of phagocytic-related factors were analyzed via qPCR and Western blot techniques. Additionally, microglia were partially depleted to verify their functional contributions. TUNEL assay and RNA sequencing were performed to further explore the underlying mechanisms. Our findings indicated that central retinal vascular regression occurs rapidly at an early stage, accompanied by microglial migration and activation. Endothelial cell fragments were detected within microglia, confirming their involvement with phagocytic function, the upregulation of phagocytic-related factors further proved it. Notably, partial depletion of microglia significantly inhibited early retinal vascular regression and reduced endothelial cell apoptosis. Moreover, RNA sequencing and bioinformatics analysis revealed that microglial phagocytosis is regulated by the complement pathway. In conclusion, our study confirms the active participation of microglia in hyperoxia-induced retinal vascular regression through phagocytosis, thereby providing potential targets for early intervention in related diseases.
-
Key words:
- Microglia /
- Retina /
- Hyperoxia /
- Phagocytosis














