-
As an important source of animal protein and other nutrients, pork holds a vital position in global meat consumption and production. China is a major producer and consumer of pork, and pig farming is the backbone of domestic animal husbandry[1]. The economic development and rising per capita income have driven the increase in pork consumption of China[2], particularly the demand for high-quality pork products. As a result, improving pork quality is now a key issue in the pig industry.
A dietary supply of amino acids is an effective approach for improving pork quality[3]. Glutamate (Glu) was one of the most abundant non-essential amino acids in animal tissues, which is regarded as an important energy substrate for the intestine and regulating energy homeostasis[4]. Rising studies have found that Glu exerted a positive effect on the growth, development, reproduction, and production performance of swine[5]. Dietary supplementation with monosodium glutamate (MSG) increased the weight of postweaning pigs in a dose-dependent manner[6]. Glu could promote muscle cell protein synthesis and alleviate muscle protein loss induced by lipopolysaccharide (LPS) treatment in piglets[7]. 1% Glu and 1% Argine significantly reduced the backfat thickness while improving the intramuscular fat (IMF) content, meat color, and fatty acid composition in the longissimus thoracis (LD) muscle of Duroc × Large White × Landrace growing-finishing pigs[8].
Shaziling pig is a local Chinese breed that has good meat quality[9]. Yang et al. found that the differentially expressed genes and proteins in the LD muscle of Shaziling pigs and Yorkshire pigs were mainly involved in fatty acid metabolism, IMF deposition, and skeletal muscle growth[9]. In our previous study, we found that 1% Glu improved carcass traits, growth performance, and IMF contents of Shaziling pigs[10]. In addition to sensory attributes, the nutritional value of the lipids is a determinant of pork quality[11]. Therefore, investigating the role of Glu in regulating the nutritional characteristics of pork is of great significance. This study aimed to examine the effects of 1% Glu on amino acids profile and lipid compositions in the muscle of Shaziling pigs, providing a theoretical basis for high-quality pork production.
-
All animal models and experimental procedures were approved by the Zhejiang University Animal Care and Committee (ZJU20240229) and the Institute of Subtropical Agriculture, Chinese Academy of Sciences (ISA-2020-023). In the experiment, 48 purebred Shaziling pigs with 150 d of age and 31.56 ± 0.95 kg weight (male, castrated) were selected and randomly divided into a control (Con) group, and a Glu group based on a single-factor experimental design. Each group had six replicates, with each replicate consisting of four pigs. The pigs were provided with a basal diet in the Con group with a basic diet with 1% Glu supplementation in the Glu group. The supplementation dosage was based on our previous studies[10,12]. The Glu level in Con and Glu-supplemented diet were 2.42% and 3.25% respectively, as reported in our earlier work[10] . The basal diet meets the nutritional requirements of pigs based on GB/T 39235-2020, detailed composition is shown in Supplementary Table S1. The experimental period was 56 d, with 5 d of pre-feeding, and 51 d of formal experimentation, pigs were given free access to food and water. Following the termination of the experimental phase, one pig from each replicate (six pigs from each group) was selected, and then fasted for 12 h before being humanely slaughtered. The carotid artery was gently incised within 3 min after slaughter, and blood was rapidly collected. After standing at 4 °C for 30 min, all samples were centrifuged (3,500 g, 4 °C, 10 min), and serum samples were aliquoted and stored (−80 °C) for subsequent studies. For the analysis of amino acid composition, fatty acid profile, and lipidomics, the LD muscle (approximately 150 g) was rapidly collected, flash-frozen in liquid nitrogen, and stored at –80 °C (CryoCube® F570, Eppendorf AG, Germany) for subsequent use.
Serum biochemical indices analysis
-
The levels of alanine aminotransferase, aspartate, aminotransferase, apolipoprotein A, apolipoprotein B, blood glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, free fatty acids, insulin, triglyceride, and total cholesterol were measured using a 7600 Automatic Biochemistry Analyzer (Hitachi, Japan).
Amino acid composition analysis
-
Targeted metabolomics based on the MRM method was used to conduct an absolute quantitative analysis of free amino acid composition in muscle tissue. A 60 mg muscle sample was weighed and 150 μL water added, after vortexing for 60 s, 800 μL methanol (Thermo Fisher Scientific, USA) / acetonitrile (Thermo Fisher Scientific, USA) solution (volume ratio 1:1) and 50 μl isotope internal standard mixed solution were added to prepare the sample. The sample was separated using the Agilent 1290 Infinity LCultra high-performance liquid chromatography system. Mass spectrometry analysis was performed in positive ion mode using a 5500QTRAP mass spectrometer (AB SCIEX). Multiquent software was utilized to determine the chromatographic peak areas and retention times. The retention times were then adjusted according to the standards of amino acids and their derivatives for metabolite identification.
Fatty acid composition analysis
-
The main experimental steps for targeted metabolomics detection based on GC-MS for absolute quantitative analysis of fatty acid composition in muscle tissue were as follows. Fifty one fatty acid methyl ester mixed standard solutions were prepared into ten working standard solutions with different mixed standard concentration gradients using n-hexane. A 50 mg muscle sample was weighed and metabolites were extracted using a chloroform (Yonghua Chemical Co., Ltd., China) / methanol (Thermo Fisher Scientific, USA) mixed solution (volume ratio 2:1). Thermo Trace 1300/TSQ 9000 gas mass spectrometry was used for mass spectrometry analysis. The chromatographic peak area and retention time were extracted using the MSD Chemstation software. Subsequently, a standard curve was drawn to calculate the concentration of medium and long-chain fatty acids present in the sample.
Lipidomics analysis
-
Untargeted lipidomics analysis of muscle tissues was performed according to our previously published study[13]. To evaluate the system's stability and data reliability, a pooled QC sample was created by combining the separate samples taken equally from each group. Fifty mg muscle sample was weighted and lipids were extracted using a 90% isopropanol (Thermo Fisher Scientific, USA) / acetonitrile (Thermo Fisher Scientific, USA) solution. LC-MS/MS analysis was performed using a Q Exactive plus mass spectrometer (Thermo Fisher Scientific, USA) coupled with a UHPLC Nexera LC-30A (SHIMADZU). Positive and negative ion modes were investigated using full-scan spectroscopy with mass-to-charge ratio (m/z) ranges of 200−1,800 and 250−1,800 respectively. Next, the mass-to-charge ratios of lipid molecules and their fragments were acquired using the following procedure: after each full scan, ten fragment patterns (MS2 scan, HCD) were recorded. For lipid identification, peak extraction, peak alignment, and quantification, LipidSearch software version 4.1 (Thermo Scientific™) was employed. The extracted ion features were restricted to include only those that exhibited at least 50% non zero measurement values across at least one experimental group.
Statistical analysis
-
The experimental data were initially processed using Microsoft Excel 2019. A two-tailed Student's t-test was performed with IBM SPSS statistics 20 to compare differences between groups. Data were represented as mean ± SEM. Statistical significance was determined as follows: * p < 0.05, ** p < 0.01 indicating significant differences. Differences were considered with trends at 0.05 ≤ p < 0.10. Data visualization was performed using GraphPad Prism (version 9.0.0) and R (version 4.4.0), with R packages ggplot2 (version 3.5.1) and ggprism (version 1.0.5).
-
This study compared a control group with an experimental group supplemented with 1% Glu. The effects of Glu supplementation were assessed by measuring the levels of serum biochemical indices related to glucose and lipid metabolism. As shown in Table 1, Glu had no significant effect on the serum levels of alanine aminotransferase, apolipoprotein A, apolipoprotein B, blood glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, free fatty acids, insulin, triglyceride, and total cholesterol (p > 0.05) of Shaziling pigs. The content of aspartate aminotransferase (p = 0.067) tended to increase.
Table 1. Effects of Glu on serum biochemical indices of Shaziling pigs*.
Items CON GLU p value ALT (U/L) 53.00 ± 11.72 59.83 ± 3.80 0.243 AST (U/L) 68.00 ± 18.06 89.67 ± 15.15 0.067 ApoA (g/L) 0.23 ± 0.02 0.23 ± 0.02 0.457 ApoB (g/L) 0.02 ± 0.004 0.02 ± 0.005 0.092 HDL-C (mmol/L) 1.24 ± 0.29 1.09 ± 0.15 0.345 LDL-C (mmol/L) 1.79 ± 0.39 1.83 ± 0.14 0.827 TG (mmol/L) 0.61 ± 0.13 0.58 ± 0.09 0.705 TChol (mmol/L) 3.20 ± 0.67 3.13 ± 0.24 0.818 NEFA (mmol/L) 0.21 ± 0.04 0.21 ± 0.05 1.000 Glu (mmol/L) 4.62 ± 0.62 4.96 ± 0.57 0.379 * Results are presented as the mean ± SEM, n = 6, number of replicates. Effects of dietary Glu supplementation on the amino acid composition of LD muscle
-
Glu-induced changes were next explored in the muscle amino acid profile of Shaziling pigs using targeted amino acid analysis. We found that Glu had no significant effect on the contents of flavor amino acid (FAA), essential amino acid (EAA), and total amino acid, as well as individual free amino acids (p > 0.05) (Supplementary Table S2).
Glu enhanced beneficial fatty acid deposition in LD muscle of Shaziling pigs
-
To explore the role of Glu in modulating the muscle fatty acid composition of Shaziling pigs, we performed targeted fatty acid analysis in LD muscle collected from the Glu and Con groups. Results are shown in Fig. 1. The results showed that, compared with the Con group, Glu significantly increased the total fatty acid content in the LD muscle of Shaziling pigs (p < 0.05) (Fig. 1a). Glu significantly increased the contents of medium chain fatty acid (MCFAs) including caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), lauric acid(C12:0), and odd-numbered fatty acids including undecanoic acid (C11:0), pentadecanoic acid (C15:0), and trans-heptadecanoic acid (C17:1T) (p < 0.05), while significantly reducing C17:1 (p < 0.05). Additionally, Glu promoted long chain fatty acid (LCFAs) accumulation in the LD muscle, including myristic acid (C14:0), myristoleic acid (C14:1), palmitic acid (C16:0), stearic acid (C18:0), cis-7-elaidic acid (C18:1n-7), oleic acid (C18:1n-9), α-linolenic acid (C18:3n-3), arachidic acid (C20:0), eicosadienoic acid (C20:2), and arachidonic acid (C20:4n-6) (p < 0.05) (Fig. 1b−d). We next analyzed the saturation of fatty acids. We found that Glu dramatically increased the content of total saturated fatty acids (SFA) (p < 0.05), monounsaturated fatty acids (MUFA) (p < 0.05) and polyunsaturated fatty acids (PUFA) (p < 0.05) in the LD muscle compared to Con group (Fig. 1e). Furthermore, Glu had no effect on the ratio of SFAs / PUFAs (p > 0.05) (Fig. 1f) and MUFAs / PUFAs (p > 0.05) (Fig. 1g), but significantly promoted the accumulation of n-3 PUFAs in the LD muscle compared to the Con group (p > 0.05) (Fig. 1h).
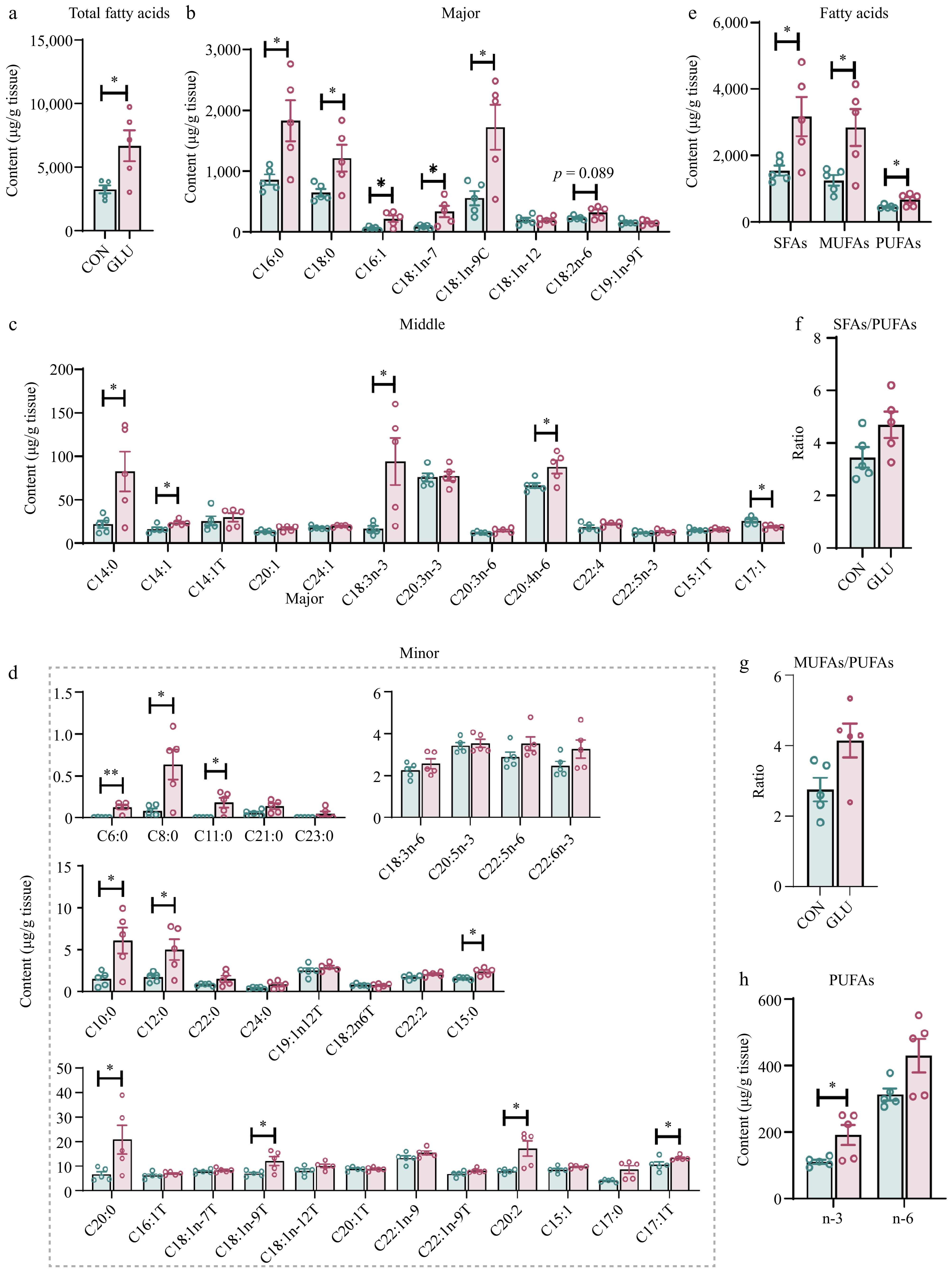
Figure 1.
Glu optimized the fatty acid profile in LD muscle of Shaziling pigs. (a) The total fatty acid content. The concentration of individual fatty acids in the LD muscle. Fatty acids are categorized into (b) major, (c) middle, and (d) minor classes based on their relative abundance, and classified by the degree of saturation. (e) Total SFAs, MUFAs, and PUFAs in muscle from Glu and Con pigs. SFAs, saturated fatty acids; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids containing two or three to six double bonds. (f) The ratio of SFA to PUFA. (g) The ratio of MUFA to PUFA. (h) The content of n-3 PUFA and n-6 PUFA. n = 5. The error bars represent the SEM. * p < 0.05, ** p < 0.01, two-tailed Student's t-test.
Glu influenced lipid composition in LD muscle of Shaziling pigs
-
To detail the effects of Glu on lipo-nutritional quality, we analyzed the lipid composition of the LD muscle from Glu and control pigs by mass spectrometry-based lipidomic analysis. Lipidomics data show that Glu had no substantial effect on the total lipid content in Shaziling pigs (Fig. 2a). The orthogonal projections to latent structure discriminant analysis (OPLS-DA) plot showed a clear separation of Glu and Con (Fig. 2b). Lipid quantitative analysis detected a total of 2124 distinct lipid molecules across 40 lipid subclasses, mainly from five lipid subclasses, including 423 triglycerides (TGs), 196 diglycerides (DGs), 180 phosphatidylcholines (PCs), 313 phosphatidylethanolamines (PEs), and 138 phosphatidylserines (PSs) (Fig. 2c). We next explored the effects of Glu on lipid subclasses composition in LD muscle of Shaziling pigs (Fig. 2d−h). Fourty lipid subclasses were classified into five categories including glycerolipids (GL), serol lipids (SL), sphingolipids (SL), glycerophospholipids (GP), and fatty acyls (FA). Compared to the Con group, Glu significantly decreased the content of GP in the LD muscle (p < 0.05) (Fig. 2g). Moreover, we analyzed the lipid subclass composition of GL, SL, and GP (Fig. 2i−k). In the GL pool, monoglyceride (MG) tended to decrease (p = 0.09) in the Glu group compared to the Con group (Fig. 2i). In the SL pool, Glu significantly decreased the content of monosialo-dihexosylganglioside (GM3) (p < 0.05) (Fig. 2j). In the GP pool, Glu induced a significant decrease in phosphatidylglycerols (PGs) (p < 0.05) and tended to decrease the contents of PI, PC, and lyo-PC (Fig. 2k). In addition, Glu elicited a significant decrease of lipid molecules in the GP pool, including PC (36:2), PC (18:3e_18:1), PE (16:0e_21:1), PG (16:0_18:1), PS (18:0_18:1) (p < 0.05), and so on (Fig. 2l).
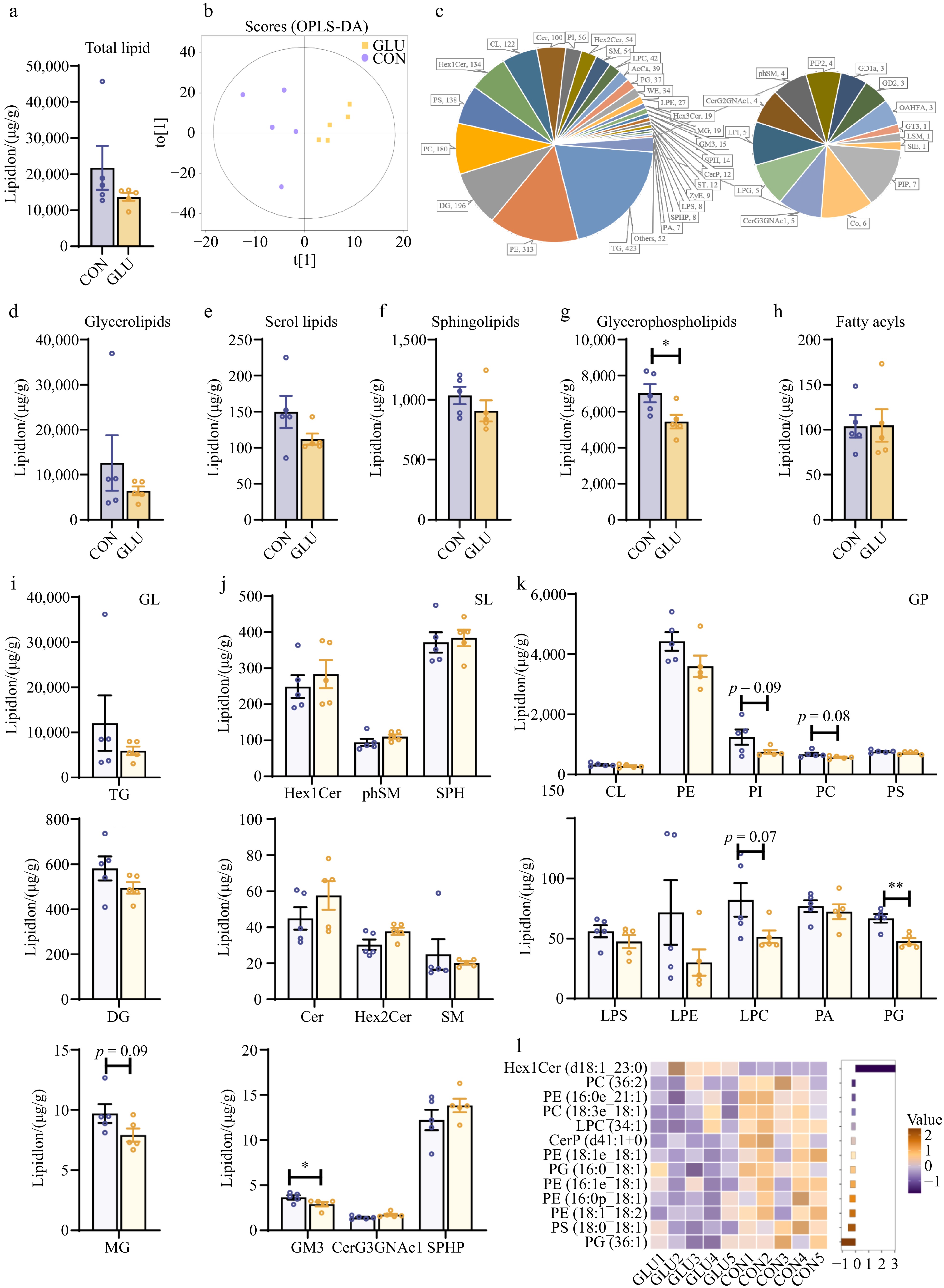
Figure 2.
Effects of Glu on the lipid composition of LD muscle of Shaziling pigs. (a) The content of total lipids. (b) The orthogonal projections to latent structures discriminant analysis (OPLS-DA) plot. (c) The types and amounts of lipids in LD muscle of Shaziling pigs. (d)−(h) Content of different lipid classes in LD muscle of Shaziling pigs. (i) The content of glycerolipids subclasses. (j) The content of sphingolipid subclasses. (k) The content of glycerophospholipids subclasses. (l) Heat map of differential lipid species in LD muscle of Shaziling pigs. n = 5. Error bars represent SEM. * p < 0.05, two-tailed Student's t-test.
Next, we analyzed the individual fatty-acyl-chain composition associated with GL GP, SL, ST, and FA (Fig. 3a−d, Supplementary Fig. S1a−b) in the LD muscle of Shaziling pigs. Compared to the Con group, Glu induced a broad decrease of lipid molecules with 0 to 10 double bonds in the GL pool (Fig. 3a), lipids with 0 to 5 double bonds in the GP pool (Fig. 3b), and lipids with 0−3 double bonds in SL pool (Fig. 3c). In the GP pool, Glu significantly decreased the content of lipid molecules with five double bonds (p < 0.01), and increased the content of lipid molecules with six double bonds (p < 0.05) (Fig. 3d). Then we explored the influence of Glu on the length of acyl chains (Fig. 3e−h, Supplementary Fig. S1c &d). Similarly, Glu induced an extensive reduction of lipid molecules in the GL pool that contain 40−60 carbons, but the total content did not reach statistical differences (p > 0.05) (Fig. 3e & h). Moreover, Glu significantly decreased the total content of lipid molecules containing 30, 31, 34, 35, and 82 (p < 0.05) carbon atoms in GPs (Fig. 3h).
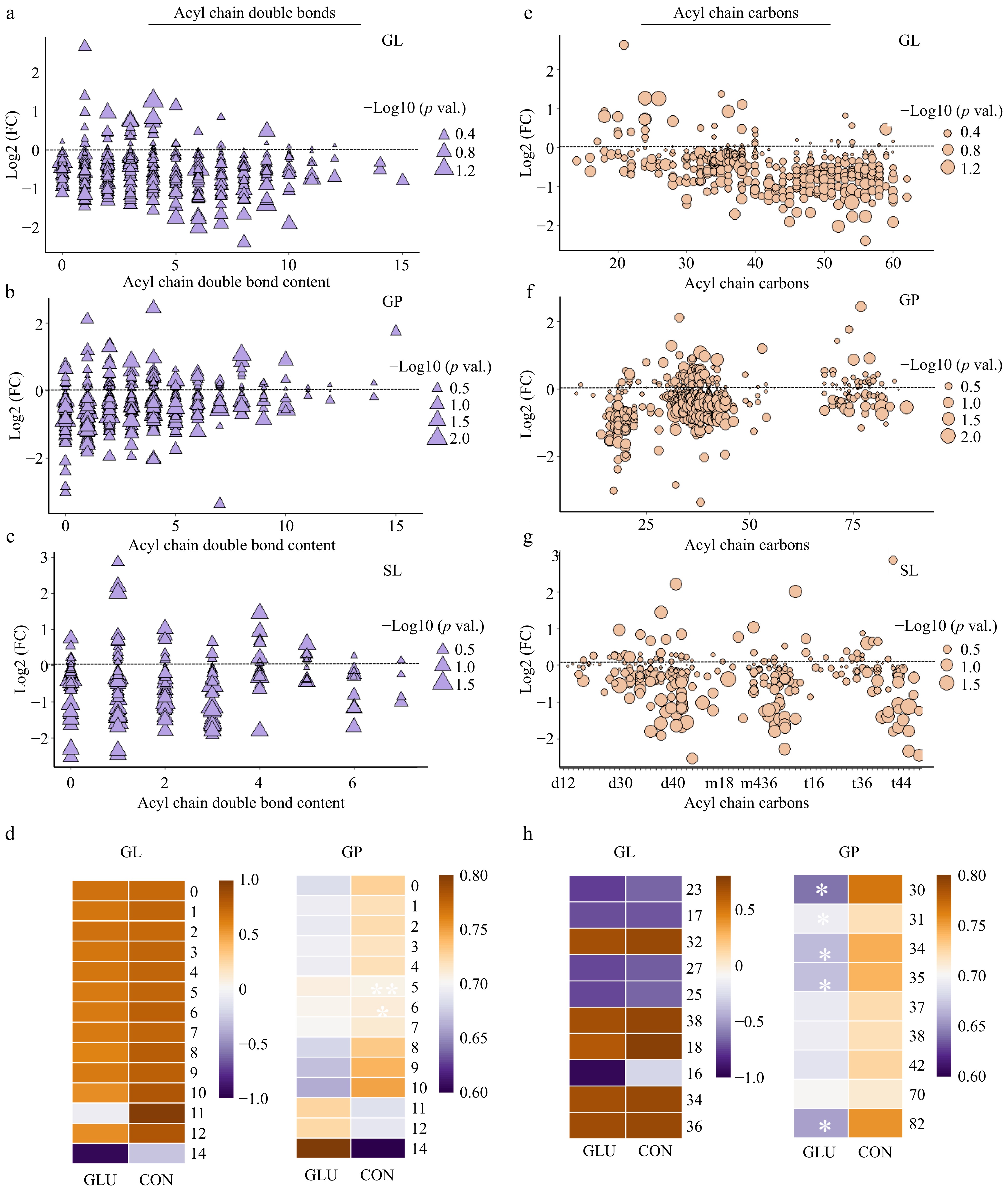
Figure 3.
Glu affected the lipid acyl chain compositions in the LD muscle of Shaziling pigs. (a) GLs with different double bond contents. (b) GPs with double bond contents. (c) SLs with different double bond contents. (d) Heatmap of acyl chain double bond contents in GL and GP pools from the control and Glu groups. (e) GLs with different numbers of carbon atoms. (f) GPs with different numbers of carbon atoms. (g) SLs with different numbers of carbon atoms. (h) Heatmap of acyl chain carbon atoms in GL and GP pools from the control and Glu groups. n = 5. Error bars represent SEM. * p < 0.05, two-tailed Student's t-test.
In this study, we found that Glu induced a significant reduction of PG and tended to decrease the total contents of PI, PC, and LPC. To figure out the role of Glu on the properties of GPs, we further analyzed its individual acyl chain composition. Although Glu did not change the overall degree of saturation of PC, it induced the decrease of C12:0, C23:0, C30:0e, C17:1, C18:1, C20:1, C27:1, C31:1, C18:3, C34:4, and C38:7 (p < 0.05) (Fig. 4a−d). In PS, Glu tended to reduce the total content of odd-number acyl chains (p = 0.08) (Fig. 4e−g), which was consistent with the reduction in C17:0 (p < 0.05) (Fig. 4h). Glu had no significant effect on PG saturation (p > 0.05) (Fig. 4i−k), but it reduced the content of C16:0, C18:0, C20:4, and C36:1 (p < 0.05) (Fig. 4l). In CL, Glu induced significant decreased of C68:5, C81:3, C82:4, 82:6, and 82:8 (p < 0.05) (Supplementary Fig. S2a). Glu exhibited modest effects on the composition of the individual acyl chains of PE, PI, and LPC in the LD muscle (Supplementary Fig. S2b−d).
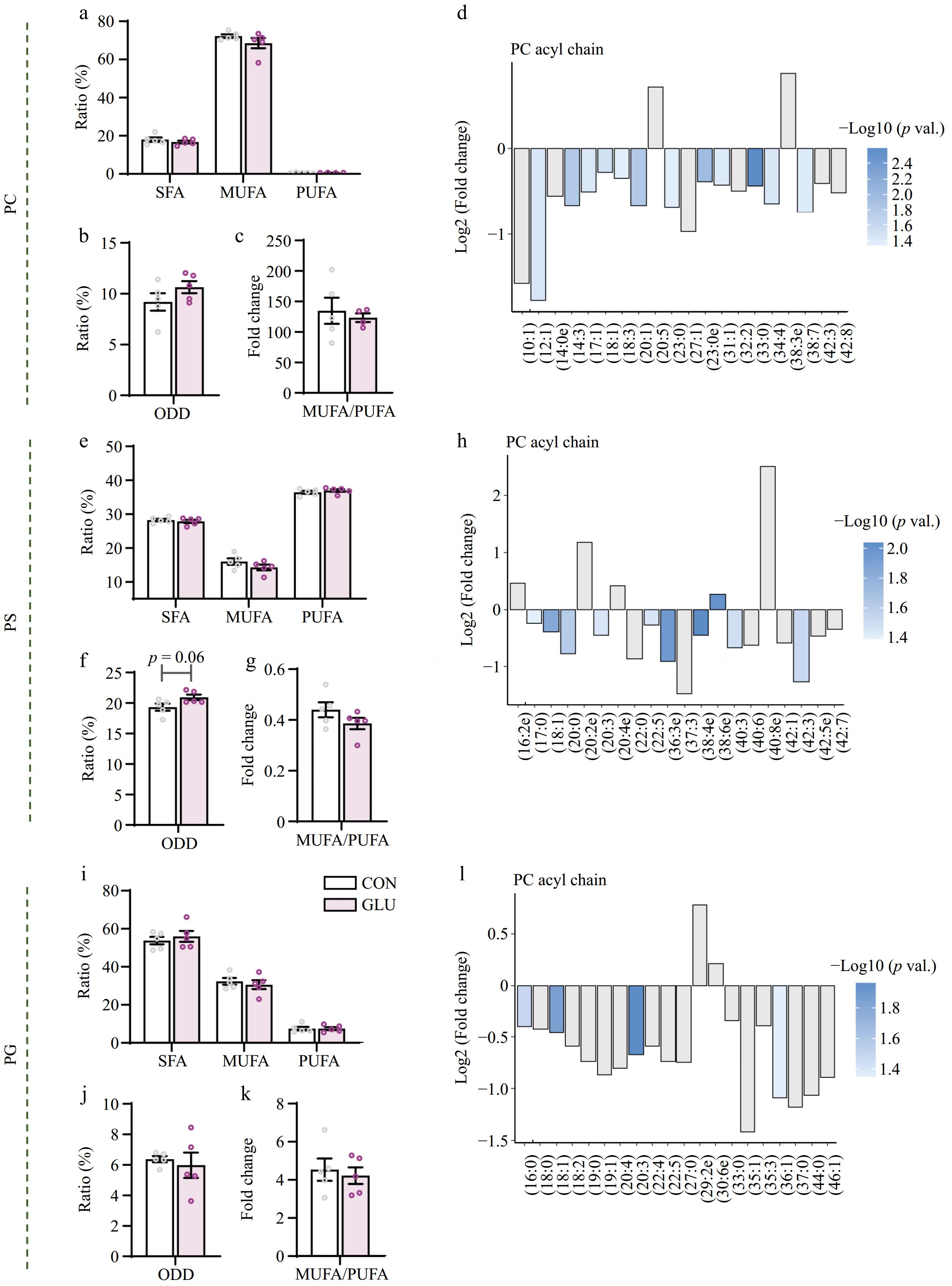
Figure 4.
Glu impacted acyl chain saturation levels of glycerophospholipid in LD muscle. (a) PC acyl chain contents at different saturation levels. (b) The content of fatty acyl chains with odd-numbered carbons in the PC pool. (c) The ratio of MUFA to PUFA in the PC pool. (d) Fold changes in the intensity of individual fatty acyl chains associated with PCs and sorted by saturation. (e) PS acyl chain contents at different saturation levels. (f) The content of fatty acyl chains with odd-numbered carbons in the PS pool. (g) The ratio of MUFA to PUFA in the PS pool. (h) Fold changes in the intensity of individual fatty acyl chains associated with PSs and sorted by saturation. (i) PG acyl chain contents at different saturation levels. (j) The content of fatty acyl chains with odd-numbered carbons in the PG pool. (k) The ratio of MUFA to PUFA in the PG pool. (l) Fold changes in the intensity of individual fatty acyl chains associated with PGs and sorted by saturation. n = 5. Error bars represent SEM. * p < 0.05, two-tailed Student's t-test.
-
This study is the first to demonstrate the impact of dietary Glu supplementation on the lipo-nutritional quality of pork in finishing pigs. Unexpectedly, Glu supplementation had minimal effects on serum biochemical parameters, and the amino acid profile of meat. However, it induced significant alterations in the fatty acid profiles and lipid compositions of pork. Specifically, 1% Glu supplementation significantly increased the levels of C18:1n-9 and 18:3n-3, and the content of PUFA in pork. Moreover, Glu influenced the muscle lipid profiles by reducing GP content. It also reshaped the individual acyl chain composition within the GL and GP pools. In summary, this study elucidates that dietary supplementation of 1% Glu can modulate the lipid nutritional quality of the LD muscle in Shaziling pigs.
Glutamate plays a crucial role in protein synthesis and various metabolic processes in the body. Previous studies reported that Glu supplementation increased serum levels of immunoglobulin A, immunoglobulin G, immunoglobulin M, globulin, and growth hormones in heat-stressed Hu sheep[14]. Glu supplementation significantly increased SOD activity and restored serum T-AOC levels and NO levels compared to the diquat group in piglets[15]. However, the effects of Glu supplementation on glucose and lipid metabolism remain unclear. In our previous study, dietary leucine supplies decreased the serum glucose levels of Shaziling pigs[12]. In this study, we found that Glu does not affect serum biochemical indices associated with glucose metabolic homeostasis, including glucose and insulin concentrations. We also addressed the effect of Glu on serum lipids and found that Glu did not affect serum lipids. These results suggested that Glu did not affect the metabolic homeostasis of Shaziling pigs. However, we found that Glu increased serum AST level, a commonly used clinical biomarker of hepatocellular injury[16], which exhibits higher activity in muscle than in the liver[17]. AST plays an important role in maintaining the NAD+/NADH ratio of cells by oxidizing NADH in the cytosol and reducing NAD+ in the mitochondrial matrix[16] , suggesting that Glu may influence AST synthesis in porcine muscle, thereby affecting the mitochondrial function of muscle.
Pork is a source of high-quality animal protein, which contains over 20 amino acids including eight essential amino acids[18]. Previous studies have reported that dietary β-alanine significantly increased the concentrations of arginine, alanine, and Glu in the LD muscle of Ningxiang pigs[19] . Moreover, a combination of 1% leucine and 1% Glu increased Glu concentrations in biceps femoris and lysine, taurine concentration in the LD muscle in fattening pigs[20] . However, some studies have reported that dietary amino acids supplementation did not reshape the muscle amino acids profile. For instance, supplementation with arginine and Glu did not affect the content of free amino acids in the LD muscle of DLY growing-finishing pigs[8] , and 1% Leu supplementation did not affect the free amino acid profile in the muscle of Shaziling pigs. In the current study, we also found that Glu did not alter the amino acids composition in the LD muscle of Shaziling pigs. This discrepancy might be attributed to various factors, such as growth stage, muscle location, breed, and amino acid synergism.
The content and composition of fatty acids affected the edible value and nutritional quality of meat[21,22]. Fatty acids in pork contribute to flavor formation, such as C18:0 and unsaturated fatty acids (UFAs) including C16:1, C17:1, C18:1n-9, and C18:2n-6[23]. The content of C18:2n-6, C20:4n-6, and C22:4n-6 in phospholipid fatty acids had a positive effect on pork flavor and overall acceptability[24]. Our results showed that Glu significantly increased the content of C16:1, C18:0, and C18:1n-9 in the LD muscle, suggesting that feeding Glu has great potential to improve the sensory quality of pork in Shaziling pigs. On the other hand, the composition of fatty acids influences the nutritional quality of pork. C20:4n-6 was rich in meat and fish products and played an important role in regulating cardiovascular function under physiological and pathological conditions[25]. As an odd-chain fatty acid (OCFAs), C17:1 might play a potential role in the pathogenesis of depressive symptoms in stroke[26], and the comorbidity of depression and coronary heart disease[27]. C18:3n-3 supplementation at a dose of ≥ 3 g/d had a more prominent effect on improving CVD risk profiles[28]. Our results showed that Glu significantly increased the content of C20:4n-6, C17:1, C18:1n-9, and 18:3n-3 in the LD muscle, suggesting that Glu has a great potential to improve the fatty acid composition of muscle in Shaziling pigs. The position of the first double bond could lead to the division of PUFA into n-3 PUFA, and n-6 PUFA[29]. n-3 PUFAs was an important dietary fatty acid known for its effects of cardio- and neuro-protection[30]. Our results showed that Glu increased the content of n-3 PUFA in the LD muscle, which improve the health benefit of pork products. The above results indicated that the supplementation of 1 % Glu can improve the nutritional quality of pork by altering its fatty acid profile.
Glycerophospholipids are assembled into bilayer membranes of mammalian cells to form the plasma membranes and organelle membranes. They serve crucial functions in a variety of cellular processes including cell growth, membrane trafficking, apoptosis, intracellular signaling, and so on[31]. In this study, discrepancy to markedly increased total fatty acids contents, Glu significantly decreased total GPs in the LD muscle of Shaziling pigs. Our previous study on Heigai pigs found that CLA improved the fat content, but not GP content in the LD muscle[13]. In line with Glu, Leu supplementation also induced a significant reduction of PE, CL, and PG, and increased total fatty acid content in the LD muscle of Shaziling pigs[12]. Similar results suggested that GP metabolism might be involved in Glu-induced accumulation of IMF and alteration of fatty acid profiles. Additionally, a previous study found that a serine-to-glycine ratio of 1:2 increased IMF and promoted the expression of genes involved in lipid oxidation in the skeletal muscle of pigs[32], indicating that lipid oxidation might contribute to Glu-induced accumulation of fatty acids in the muscle. We further analyzed the composition of the lipid subclass, which can reflect changes in lipid function[33]. We found that Glu significantly decreased the content of GPs and two differential lipid subclasses including GM3 and PG. A previous study investigated the potential associations between GPs and major meat quality traits during storage, suggesting that higher contents of PS and CL in beef were correlated with enhanced muscle oxidation, reduced shear force, and water-holding capacity[34]. In this context, the Glu-induced-reduction of total GPs might have a positive effect on the meat quality of Shaziling pigs. Additionally, GM3 plays a role in transmitting immune signals during viral infections, and GM3 lipids have been associated with the development of atrial fibrillations[35]. PG is a mitochondrial phospholipid and a precursor of cardiolipin (CL), a dynamic functional marker of mitochondria, which is linked to various metabolic diseases[36]. After re-biosynthesis in the mitochondria, PG would be remodeled by binding the appropriate acyl content to perform its biological function and prevent the detrimental effects of lysophosphatidylglycerol (LPG) accumulation[37]. The reduced GM3 content suggested that pigs in the Glu treatment group were healthier than those in the Con group. Additionally, the decreased PG level indicated that compared with the Con group, mitochondria biosynthesis was more active in the muscle of the Glu group. Glu also induced a significant decrease in several lipid species in the GL pool of LD muscle. Notably, PC (36:2) is negatively associated with hepatic TG accumulation in pigs[38]. PS (18:0/18:1), which is enriched in the inner leaflet of cellular membranes, plays a critical role in various cellular processes, including protecting cholesterol from cholesterol oxidase and clustering with cholesterol in liposomes[39,40]. These findings suggest that Glu has the potential to reduce TC content in LD muscle. Given these results, we hypothesized that Glu may influence muscle mitochondria function, promoting GPs oxidation and fatty acid absorption in the LD muscle of Shaziling pigs. In the present study, Glu induced a great reduction of total GP and lipid species in the GP pool. Since GPs had both positive and negative influences on the volatile level[41], the effect of Glu-induced reduction in GPs content on meat flavor remains to be further evaluated.
GPs consist of three building blocks that include a polar head, a central group, and a long hydrocarbon chain[42]. While the polar heads of lipids have received much attention for their specific interactions with proteins, the roles of the various fatty acyl chains in phospholipids (PL) remain incompletely understood[42]. Recent studies showed that acyl chains have diverse structures that influence the elongation, desaturation, and transport of fatty acids[43]. A previous study showed that fermented mulberry leaves improve meat quality mainly by suppressing TG deposition and modulating the synthesis of long-chain fatty acids in the muscle tissue of Yuxi black pigs[32]. In this study, we also observed decreased TGs with long acyl chains. Consistent with a previous study that found a reduction in phospholipids with 4−6 double bonds in the PC, PS, and PI pools during myogenic differentiation[44], we observed that Glu significantly reduced GPs containing six double bonds, which may be associated with muscle development in Shaziling pigs. Additionally, the concentration of OCFAs in human plasma phospholipids is negatively associated with cardiovascular disease[45]. In this study, we showed that Glu increased OCFA content in the PS pool, which may enhance the nutritional quality of meat products.
-
The current study showed that dietary supplementation with 1% Glu had negligible effects on the levels of serum biochemical indices and the muscle amino acid profile in Shaziling pigs. Conversely, it significantly affected the lipo-nutritional quality of the LD muscle. Glu significantly increased the content of C16:1, C18:0, 18:1n-9c, C18:3n-3, and C20:4n-6, and markedly increased the content of n-3 PUFA. In addition, lipidomics analysis demonstrated that Glu significantly reduced GP content and affected the composition of acyl chains in the GL and GP pools. As a functional amino acid, Glu might be involved in the regulation of lipid metabolism in the muscle of Shaziling pigs, thus affecting its lipo-nutritional quality, and the specific mechanism needed further exploration.
The project was partially supported by the National Natural Science Foundation of China (U19A2037) and the 'Hundred Talents Program' funding from Zhejiang University to TZS and we thank members of the Shan Laboratory for their comments and support.
-
All procedures were reviewed and preapproved by the Zhejiang University Animal Care and Committee (identification number: ZJU20240229, approval date: 2024-4-22) and the Institute of Subtropical Agriculture, Chinese Academy of Sciences (dentification number: ISA-2020-023, approval date: 2020-9-16). The research followed the 'Replacement, Reduction, and Refinement' principles to minimize harm to animals. This article provides details on the housing conditions, care, and pain management for the animals, ensuring that the impact on the animals is minimized during the experiment.
-
The authors confirm contribution to the paper as follows: data curation, writing-original draft: Huang Y; investigation: Huang Y, Wang L, Zhang S, Zheng C, Duan Y; methodology: Huang Y, Wang L, Zheng C, Duan Y, Wang T, Zhou Y; visualization: Huang Y, Zhou Y; writing - review & editing: Wang L, Zhou Y, Shan T; conceptualization, supervision: Zhou Y, Shan T; funding acquisition, project administration, resources: Shan T. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available on request from the corresponding author.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Formula and nutrient composition of basal diet.
- Supplementary Table S2 Effects of Glu on amino acid content in the longissimus thoracis of Shaziling pigsa.
- Supplementary Fig. S1 The lipid acyl chain compositions of STs and FAs in the longissimus thoracis in Shaziling pigs. (a) STs with different numbers of double bond contents. (b) FAs with different numbers of double bond contents. (c) STs with different numbers of carbon atoms. (d) FAs with different numbers of carbon atoms.
- Supplementary Fig. S2 The acyl chain saturation levels of glycerophospholipids in the longissimus thoracis. (a) The intensity fold changes of individual fatty acyl chains associated with CLs sorted by the degree of saturation. (b) The intensity fold changes of individual fatty acyl chains associated with PEs sorted by the degree of saturation. (c) The intensity fold changes of individual fatty acyl chains associated with PIs sorted by the degree of saturation. (d) The intensity fold changes of individual fatty acyl chains associated with LPCs sorted by the degree of saturation.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Huang Y, Wang L, Zhang S, Zheng C, Duan Y, et al. 2025. Effect of dietary glutamate supplementation on the lipo-nutritional quality of pork in Shaziling pigs. Animal Advances 2: e006 doi: 10.48130/animadv-0025-0004
Effect of dietary glutamate supplementation on the lipo-nutritional quality of pork in Shaziling pigs
- Received: 14 November 2024
- Revised: 19 December 2024
- Accepted: 16 January 2025
- Published online: 03 March 2025
Abstract: Glutamate (Glu) is a major component of food proteins and is extensively utilized in livestock production. Our previous study demonstrated that dietary Glu supplementation improved the carcass traits of Shaziling pigs. However, the role of Glu in regulating the nutritional quality of pork remains unclear. This study aimed to investigate the effect of Glu on serum biochemical indices, amino acid composition, fatty acid composition, and lipid profiles in pork from Shaziling pigs. A total of 48 Shaziling pigs (150 days old, 31.56 ± 0.95 kg) were randomly divided into a control (Con) group (basic diet), and a Glu group (basic diet supplemented with 1% Glu) for a 51-d feeding trial, and six pigs per group were slaughtered for analysis. Glu had no significant effects on serum biochemical indices and amino acid profiles of the longissimus thoracis (LD) muscle. Fifty distinct fatty acids and 2,124 unique lipid molecules were identified by fatty acid and lipidomics profiling. Glu significantly increased the total fatty acids, particularly polyunsaturated fatty acids (PUFAs) in the LD muscle, and remarkably enhanced the deposition of oleic acid (C18:1n-9) and α-linolenic acid (C18:3n-3). Glu dramatically reduced the levels of monosialo-dihexosylgangliosides (GM3s) and phosphatidylglycerols (PGs), and affected multiple lipid molecules, including phosphatidylcholine (PC) (36:2), PC (18:3e_18:1), phosphatidylethanolamine (PE) (16:0e_21:1), PG (16:0_18:1), and phosphatidylserine (PS) (18:0_18:1). Additionally, Glu induced a reconfiguration of the individual acyl chain composition in the glycerolipids (GL) and glycerophospholipid (GP) pools. Overall, this study elucidated the effects of 1% Glu on the lipo-nutritional quality of LD muscle, offering insights for enhancing pork value in indigenous Chinese pig breeds.
-
Key words:
- Amino acids /
- Shaziling pigs /
- Longissimus thoracis /
- Fatty acids /
- Lipidomics














