-
Neuroendocrine tumors (NETs) arise from peptidergic neurons and neuroendocrine cells that are distributed throughout the body[1−3]. These tumors are highly heterogeneous and exhibit a spectrum of growth patterns, ranging from slow growth to high invasion and metastasis[4−6]. Over the past 30 years, the incidence rate of NETs has increased nearly sevenfold, significantly outpacing the growth rates of other malignant tumors[7]. As a result, their prevalence has surpassed that of gastric, liver, and esophageal cancers, positioning NETs as the fourth most common malignant tumor[8]. The advent of early detection methods, such as endoscopy, has led to a gradual increase in the detection rate of rectal neuroendocrine tumors (rNETs), which now rank among the top three NETs in China in terms of incidence and prevalence[9,10]. Based on the Ki67 index, rNETs can be classified into three grades: G1, G2, and G3. Among these, G1 grade rNETs are associated with a favorable prognosis, whereas G2 and G3 grade rNETs demonstrate a greater propensity for malignancy[11]. The most common metastatic sites for rNETs are the lymph nodes and liver[12]. The liver is the most common site of metastasis for well-differentiated rNETs. The liver's vascular architecture is characterized by a widespread distribution of blood vessels and a dual blood supply from both the hepatic portal vein and hepatic artery[13,14]. Given that rNETs are tumors with a rich blood supply, they are particularly prone to metastasizing to the liver[15]. Liver metastases are often diffuse and multiple, frequently exhibiting less differentiation than the primary lesions, and can even exceed the size of the primary tumors. Notably, nearly all rNETs with a diameter of 2 cm or greater have the potential to develop liver metastases. Furthermore, abdominal lymph node metastasis represents another significant pathway for rNETs spread. The presence of lymph node metastasis is associated with increased tumor malignancy and a poorer prognosis[16,17]. Research conducted by Sohn et al. indicates that the lymph node metastasis rate for G1 grade rNETs is 6%, while this rate escalates to 75% for G2 grade rNETs[18]. The majority of rNETs are classified as G1 grade, with G2 grade and G3 grade comprising only 2% to 13%, both of which are recognized as risk factors for lymph node and liver metastasis[18,19]. Currently, the guidelines recommend surufatinib and everolimus, a targeted therapy for well-differentiated metastatic rNETs, which have shown improvements in progression-free survival. However, no significant enhancement in overall survival has been observed. Poorly differentiated rNETs are primarily treated with a combination of platinum and etoposide chemotherapy, resulting in a shorter survival duration[20,21]. Once rNETs metastasize, treatment options become limited, severely impacting patient prognosis. Tumor grade and metastasis are key factors in clinical decision-making for rNETs. Consequently, identifying biomarkers for the diagnosis of rNETs holds significant research value for predicting prognosis and facilitating early intervention to improve outcomes.
Most patients with rNETs are diagnosed incidentally during colonoscopy. Advances in endoscopic and imaging techniques provide patients with the opportunity for early detection of rNETs, which can enhance surgical options and improve prognosis. However, endoscopic screening remains underutilized in many regions, and distinguishing the morphology of rNETs from rectal polyps during endoscopy can be challenging, leading to potential missed diagnoses and misdiagnoses. Consequently, serological testing plays a crucial role in the diagnostic process. Current research indicates that neurone-specific enolase (NSE) possesses diagnostic value for rNETs[22]. However, the diagnostic significance of other common tumor markers, such as carcino-embryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), for rNETs remains uncertain. This study primarily investigates the diagnostic utility of CEA, CA19-9, and NSE for rNETs, to establish a foundation for the early diagnosis of these neoplasms.
-
A total of 261 patients with rNETs admitted to Nanjing Hospital of Traditional Chinese Medicine and Jiangsu Province Hospital (The First Affiliated Hospital with Nanjing Medical University) from January 2021 to December 2024 were included in the experimental group. The inclusion criteria were as follows: rNETs had to be pathologically confirmed, all pathological sections required immunohistochemical staining, pathological data needed to be complete, the primary site had to be in the rectum, and examinations of CEA, CA19-9, and NSE had to be complete within one month before surgery. The exclusion criteria included: tumors that had metastasized from other sites to the rectum, the presence of other tumors at the time of diagnosis, the absence of immunohistochemical staining on pathological sections, and incomplete blood data for CEA, CA19-9, and NSE within one month before surgery.
Methods
-
Data from rNETs patients were collected, which included gender, age, pathological grade, presence or absence of metastasis, and blood levels of CEA, CA19-9, and NSE at the time of diagnosis. The pathological classification of rNETs is categorized into three grades: G1, G2, and G3. The normal reference value ranges for each marker are as follows: CEA ≤ 5 ng/mL, CA19-9 ≤ 30 U/mL, and NSE ≤ 16.3 ng/mL. Values exceeding the upper limit of these normal reference ranges are considered abnormally elevated.
Statistical processing
-
SPSS22.0 software was used for data analysis. Count data were expressed as the number of cases, and χ2 test was used for comparison between groups. After the normality test, the measurement data of normal distribution were expressed as mean ± standard deviation (x ± s), and an independent sample t-test was used for comparison between the two groups. The measurement data that did not conform to the normal distribution were represented by the median (first quartile, third quartile) [M (P25, P75)], and the Mann-Whitney U test was used to compare the two groups. Logistic regression was used to analyze factors affecting the grade and metastasis of rNETs, a joint diagnostic model was established, and a receiver operating characteristic (ROC) curve was drawn to analyze the diagnostic value of single and combined CEA, CA19-9, and NSE detection for predicting rNETs. p < 0.05 was considered as a statistically significant difference.
-
Among the 261 patients with rNETs, the age range was 24 to 86 years (51.68 ± 11.67). Of these patients, 152 were male and 109 were female. Additionally, 191 patients were classified as G1, 49 as G2, and 21 as G3. There were 50 cases of metastasis, while 211 cases did not exhibit metastasis. Among the patients with metastasis, 26 had liver metastasis only, 10 had lymph node metastasis only, four had both liver and lymph node metastasis, and 10 had extrahepatic metastasis. Female and aged patients were more likely to develop G2/G3 and metastasize (Tables 1, 2).
Table 1. Characteristics of rNETs patients with different pathological grade.
G1 G2/G3 p Sex (male/female) 121/70 31/39 0.006 Age (years) 50.46 ± 11.56 55.03 ± 11.39 0.005 CEA 1.78 (1.19, 2.48) 2.68 (1.35, 4.52) 0.154 CA19-9 10.15 (6.53, 13.47) 11.98 (7.17, 18.05) 0.289 NSE 15.87 (11.48, 19.75) 21.24 (16.49, 45.46) <0.001 Table 2. Characteristics of rNETs patients with or without metastasis.
Without metastasis With metastasis p Sex (male/female) 130/81 22/28 0.023 Age (years) 50.11 ± 11.99 58.32 ± 7.13 < 0.001 CEA 1.76 (1.17, 2.55) 2.78 (1.38, 5.21) 0.011 CA19-9 10.17 (6.53, 13.47) 12.00 (7.17, 25.25) 0.551 NSE 16.07 (11.74, 19.91) 20.45 (16.13, 46.07) < 0.001 Comparison of rNETs tumor marker levels between G1 and G2/G3 groups
-
In the G1 group, there were eight, six, and 88 cases respectively with CEA, CA19-9, and NSE levels exceeding the upper limit of the normal reference values, and in the G2/G3 group, there were nine, seven, and 48 cases respectively. The Mann-Whitney U test results showed that the NSE level in the G2/G3 group was higher than that in the G1 group, and the difference was statistically significant (p < 0.05), while there was no statistically significant difference in the CEA and CA19-9 levels (p > 0.05) (Table 1).
Comparison of rNETs tumor marker levels between non-metastasis and metastasis groups
-
In the non-metastasis group, the number of cases exceeding the upper limit of normal reference values for CEA, CA19-9, and NSE were eight, four, and 97 respectively. In the metastasis group, these numbers were nine, nine, and 39 respectively. The results of the Mann-Whitney U test indicated that the CEA and NSE levels in the metastasis group were significantly higher than those in the non-metastasis group, with statistically significant differences (p < 0.05), while there was no statistical difference in CA19-9 levels (p > 0.05) (Table 2).
Binary logistic regression analysis
-
The indicators that demonstrated statistically significant differences in the single-factor analysis were incorporated into the binary logistic regression analysis. Assigned a value to binary variables, grade (G2/3 = 1, G1 = 0), metastasis (yes = 1, no = 0), and gender (female = 1, male = 0). Each continuous variable was assigned its original value. Binary logistic regression analysis was conducted to derive joint prediction models to predict the grade and metastasis of rNETs. The model to predict grade is expressed as Y = 0.820 × gender + 0.029 × age + 0.087 × NSE − 4.598. The model to predict metastasis is expressed as Y = 0.772 × gender + 0.066 × age + 0.095 × NSE − 7.366.
ROC curve analysis
-
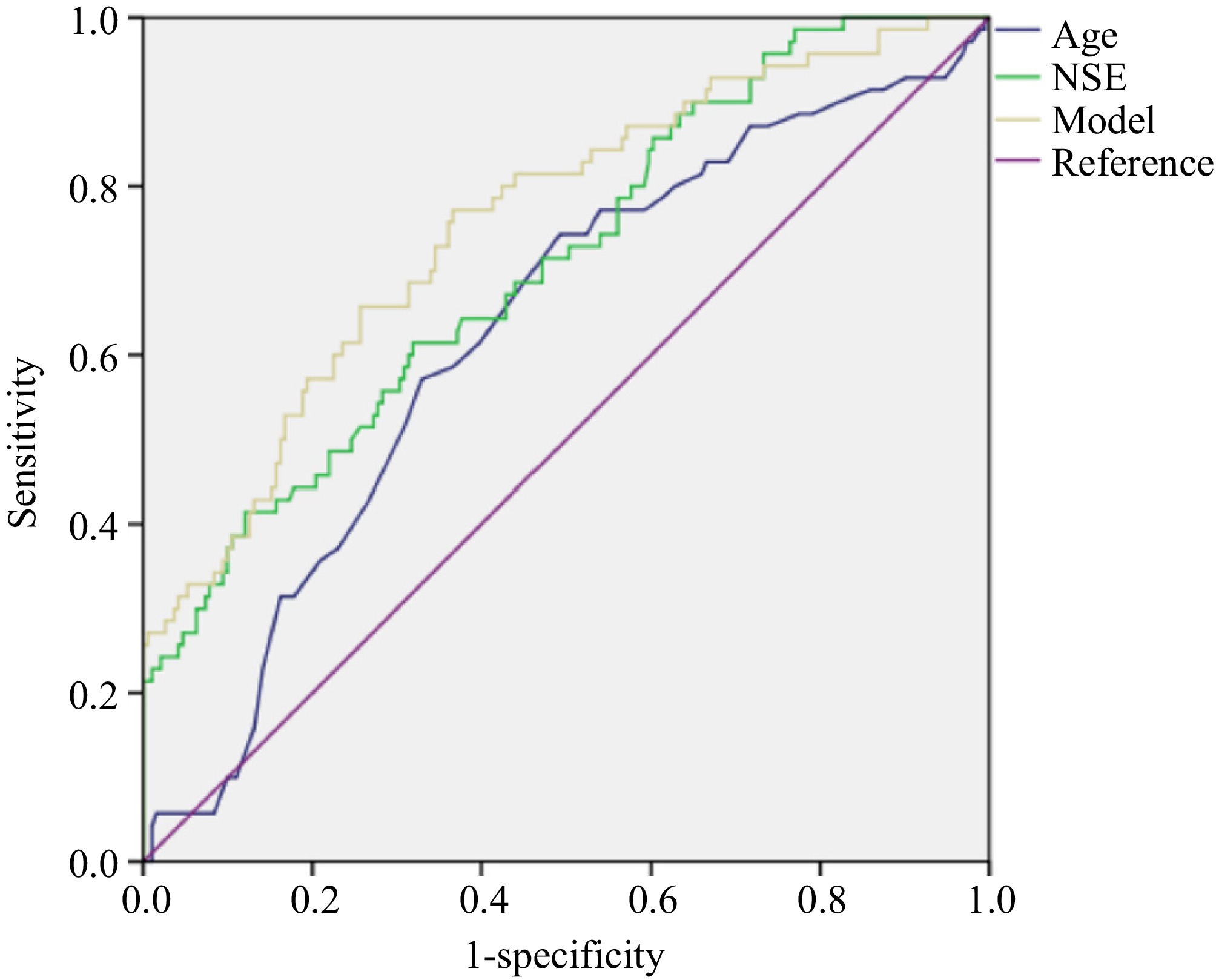
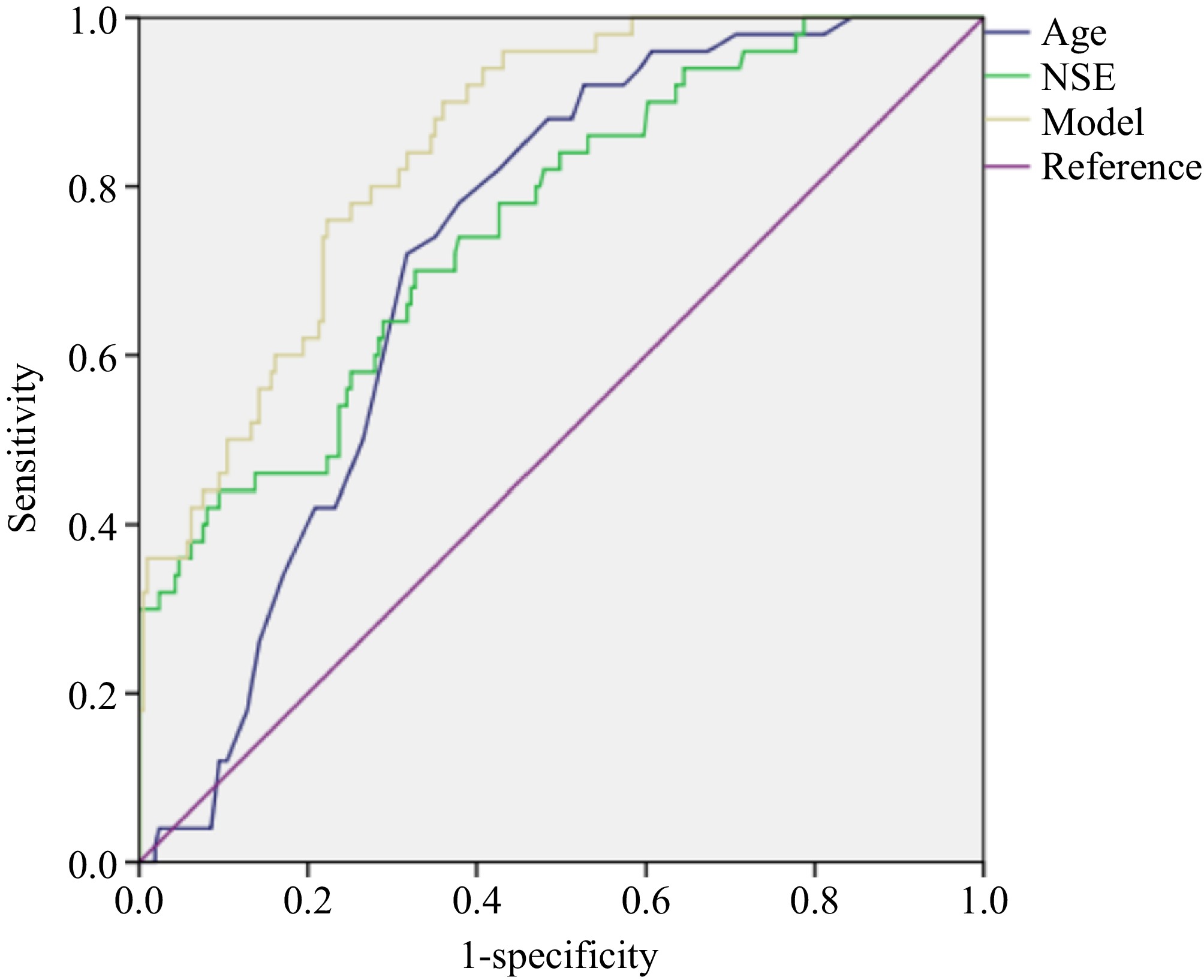
We drew ROC curves to evaluate the diagnostic value of age, NSE, and joint models to predict the grade and metastasis of rNETs. The areas under the curve (AUCs) for age and NSE in predicting grade were 0.626 and 0.708, respectively, both lower than the AUC (0.756) of the model (Table 3 & Fig. 1). The AUCs for age and NSE in predicting metastasis were 0.723 and 0.757, respectively, both lower than the AUC (0.847) of the model (Table 3 & Fig. 2).
Table 3. Results of ROCs to predict grade and metastasis of rNETs.
rNETs Index Sensitivity Specificity AUC 95% CI of AUC Cut-off
valueGrade Age 0.743 0.508 0.626 [0.550, 0.702] 51.500 NSE 0.614 0.681 0.708 [0.638, 0.779] 17.950 Model 0.771 0.634 0.756 [0.689, 0.823] 0.211 Metastasis Age 0.720 0.682 0.723 [0.658, 0.788] 55.500 NSE 0.700 0.673 0.757 [0.684, 0.830] 17.945 Model 0.900 0.640 0.847 [0.796, 0.899] 0.125 -
RNETs are clinically uncommon and exhibit significant heterogeneity[23]. Most patients present with atypical symptoms, making diagnosis challenging and often overlooked. Pathological examination remains the gold standard for diagnosis; however, there are limited serum tumor markers associated with rNETs[24]. Aside from chromogranin A (CgA) and NSE, which have demonstrated some diagnostic utility, the effectiveness of other tumor markers remains uncertain. CEA is a glycoprotein present during embryonic development, with its expression significantly suppressed after birth[5,25]. Consequently, serum CEA levels in healthy adults are typically low, but it can be overexpressed in patients with digestive tract tumors, inflammatory bowel disease, and other conditions. CA19-9 serves as a crucial serum marker for malignant tumors of the digestive system and holds particular diagnostic significance for pancreatic cancer[26]. NSE, an acidic protease specific to neurons and neuroendocrine cells, also provides valuable diagnostic information for neuroendocrine tumors[27].
This study primarily investigates the expression levels of CEA, CA19-9, and NSE in patients with different pathological types and metastasis of rNETs, as well as the diagnostic value of both individual and combined detection methods for rNETs. The results indicate that NSE levels are significantly elevated in patients with rNETs, especially in G2/G3 patients, whereas CEA and CA19-9 levels do not show a similar increase. CEA and NSE levels are significantly elevated in rNETs patients with metastasis. NSE is the common risk factor for predicting the grade and metastasis of rNETs. Previous studies have identified pathological grade and metastasis as independent risk factors influencing the prognosis of rNETs. Consequently, it is essential to examine the effect of NSE on prognosis. Interestingly, our study revealed differences related to age and gender. While male patients constituted a larger portion of the sample, the incidence of higher pathological grades and metastasis was notably greater among elderly women. This discrepancy may be attributed to the small sample size and potential selection bias. We still suggest however that women over the age of 60 should be prioritized as a key population for screening. This study has certain limitations. Firstly, the sample size is relatively small, which may introduce bias in the results. Secondly, being a retrospective study, it includes a limited range of tumor markers, thus failing to adequately explore the diagnostic value of CgA and others for rNETs. Current research indicates that emerging serum markers, such as multigene mRNAs (NETest), possess significant diagnostic value for rNETs and may facilitate earlier detection of tumors[28]. Future investigations should be done to explore the diagnostic potential of these new markers to enhance early diagnosis capabilities.
In summary, the results of this study indicate that the elevated levels of NSE is a significant risk factor for the occurrence and progression of rNETs. Future multi-center prospective studies are necessary to validate these findings, ultimately providing a theoretical foundation for the early diagnosis and treatment of rNETs.
-
The study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the ethical review committee of The First Affiliated Hospital with Nanjing Medical University, identification number: 2023-SR-310, approval date: 2023-05-31.
-
The authors confirm contribution to the paper as follows: study design and data review: Ye M, Jia X; statistical analysis and draft manuscript preparation: Zhang J; patient treatment and data collection: Zhang J, Yu Q, Zeng X; manuscript revision: Ye M, Jia X. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author upon request.
-
We would like to express our gratitude to all patients and colleagues.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This articleis an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang J, Yu Q, Zeng X, Jia X, Ye M. 2025. Diagnostic value of NSE in predicting the grade and metastasis of rectal neuroendocrine tumors. Gastrointestinal Tumors 12: e012 doi: 10.48130/git-0025-0012
Diagnostic value of NSE in predicting the grade and metastasis of rectal neuroendocrine tumors
- Received: 17 January 2025
- Revised: 17 April 2025
- Accepted: 29 May 2025
- Published online: 26 June 2025
Abstract: This study aimed to explore the diagnostic value of carcino-embryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and neurone-specific enolase (NSE) in rectal neuroendocrine tumors (rNETs). A total of 261 rNETs patients admitted to Nanjing Hospital of Traditional Chinese Medicine and Jiangsu Province Hospital from January 2021 to December 2024 were collected. Among rNETs patients with varying pathological grades, the differences in gender, age, and NSE levels were statistically significant. Aged females and elevated levels of NSE were identified as risk factors for predicting G2/G3 rNETs. Among rNETs patients with and without metastasis, the differences in gender, age, CEA, and NSE levels were statistically significant. Aged females and elevated levels of NSE were identified as risk factors for predicting rNETs metastasis. Binary logistic regression analysis was conducted to derive joint prediction models to predict the grade and metastasis of rNETs. The areas under the curve (AUCs) for age and NSE in predicting grade were 0.626 and 0.708, respectively, both lower than the AUC (0.756) of the model. The AUCs for age and NSE in predicting metastasis were 0.723 and 0.757, respectively, both lower than the AUC (0.847) of the model. In conclusion, NSE demonstrates notable diagnostic value for predicting the grade and metastasis of rNETs.
-
Key words:
- Rectal neuroendocrine tumors /
- NSE /
- Grade /
- Metastasis