-
Pitaya, also known as dragonfruit, is rich in phytochemicals that provide health benefits, such as polyphenols, flavonoids, betalains, and vitamin C, all recognized for their antioxidant effects[1]. Polyphenols help neutralize free radicals, reducing oxidative stress, while flavonoids protect cells and reduce inflammation. Vitamin C strengthens the immune system and protects cells from damage[2−6]. These bioactive compounds give pitaya a high nutraceutical potential, making it a valuable functional food for promoting health and preventing diseases.
Therefore, these bioactive compounds are essential in the human diet due to their antioxidant effects, which vary according to the structure, number, and position of the hydroxyl groups[7−9]. In this regard, different approaches and techniques can be used for the determination and quantification of these valuable constituents[10−11]. A bioactive extract is a preparation obtained from a biological matrix, such as organisms, plants, or, in the case of this study, fruit pulp, which contains compounds with specific biological activity[12]. However, the literature does not present a consensus on a standard extraction method or the most efficient solvent. Still, several studies indicate that solid-liquid extraction using different types of solvents is the most effective approach[13].
The process of obtaining a bioactive extract involves extraction and purification steps, and it is crucial to consider the purpose of the extract, as the yield and final composition are closely linked to the characteristics of the solvent used, making it a key factor. The type and method of solvent use are of utmost importance. Commonly used solvents include acetone, ethanol, and methanol, as well as water and other petroleum-derived products. The protocols for obtaining the extract typically involve the disruption of organelles, agitation, and temperature variation, which can be applied to isolate and concentrate these bioactive compounds[14,15]. The choice of extraction method is crucial for determination, as it directly influences the quantity and quality of the bioactives obtained in the final extract. Therefore, for extractions, the choice of solvents has increasingly aligned with the principles of green chemistry, which involves the use of renewable sources instead of petroleum-derived products[16].
Although many extraction protocols use isolated solvents, these methods are not always efficient, which motivates the use of combinations of different solvents. These mixtures can significantly improve the extraction of bioactive compounds[17], providing better results. An example of this is the use of water, which has the ability to increase extraction yield by promoting the swelling of the biological material, thus expanding the contact surface between the matrix and the solvent[18−20].
Recent studies have highlighted the effectiveness of optimizing solvent combinations to enhance the extraction of phenolic and antioxidant compounds from fruits and other plant-based matrices. For example, Koraqi et al.[21] optimized the extraction of these compounds from strawberries using different solvent combinations and the response surface methodology. Similarly, Rodrigues et al.[22] performed solvent screening and optimization for the extraction of phenolic compounds from grape pomace, demonstrating the high potential of mixtures such as ethanol and acetone to maximize bioactive compound yields. Despite these advances, studies specifically focused on pitaya remain scarce, especially considering the diversity of cultivars and their distinct phytochemical profiles.
According to the authors' knowledge, there are no studies on specific mathematical approaches to optimize solvent mixtures aimed at creating extracts rich in bioactive compounds from different pitaya cultivars. In this context, the use of a combination of solvents may be an effective alternative to optimize the extraction process. Therefore, the objective of this study was to optimize the extraction of bioactive compounds by employing different solvents in combination across various pitaya cultivars.
-
To optimize the extraction of bioactive compounds from pitaya (Selenicereus sp.), three cultivars were tested: 'Boreal Red', 'Chinesa', and 'Roxa do Pará' (Fig. 1), collected at the Nascente da Pitaya & Cia site, located in Bambuí – Minas Gerais, Brazil (latitude: 20°00'21'' S; longitude: 45°58'37'' W). The fruits were harvested at commercial maturity (half green, half pink scales) and selected on the basis of the external appearance, discarding those with pathogens, parasites, or defects. After selection, fruits were transported in hermetic expanded polystyrene boxes with dry ice to the Fruit and Vegetable Postharvest Pilot Plant of the Federal University of Lavras (UFLA), Brazil. Upon arrival, fruits were washed under running water, immersed in a sodium hypochlorite solution (1% v/v) for 2 min, rinsed with distilled water to remove sanitizer residues, and dried at room temperature. Subsequently, fruits were manually peeled, and the pulp was homogenized using sanitized stainless steel utensils. The homogenized samples were immediately subjected to the extraction procedures for bioactive compounds.
Figure 1.
Pitaya cultivars (Selenicereus sp.) (a) 'Boreal Red', (b) 'Chinesa', and (c) 'Roxa do Pará'.
These cultivars were selected on the basis of their commercial relevance, genetic diversity, and known differences in morphological traits and bioactive compound profiles reported in previous studies. This selection allowed for a comparative analysis to understand how genotypic variability affects the extraction efficiency and potential applications of bioactive extracts of pitaya.
Experimental design
-
The selection of solvent formulations aimed to optimize the extraction of antioxidants and phenolic compounds from pitaya. Initial tests were conducted using four solvents, namely ethanol, methanol, acetone, and water, in pure and combined concentrations. According to the extraction results for total phenolic content and antioxidant capacity, binary aqueous mixtures were generally more effective than pure solvents or nonaqueous combinations. A set of specific solvent formulations was then selected for a comparative evaluation. The extraction efficiency of each formulation was statistically analyzed and represented as the relative extraction efficiency, using analytical procedures in R, allowing the identification of the most effective solvent combinations for phenolic and antioxidant extraction from pitaya, as illustrated in Fig. 2. This approach provided a practical and statistically robust method to optimize the extraction conditions without requiring exhaustive factorial designs.
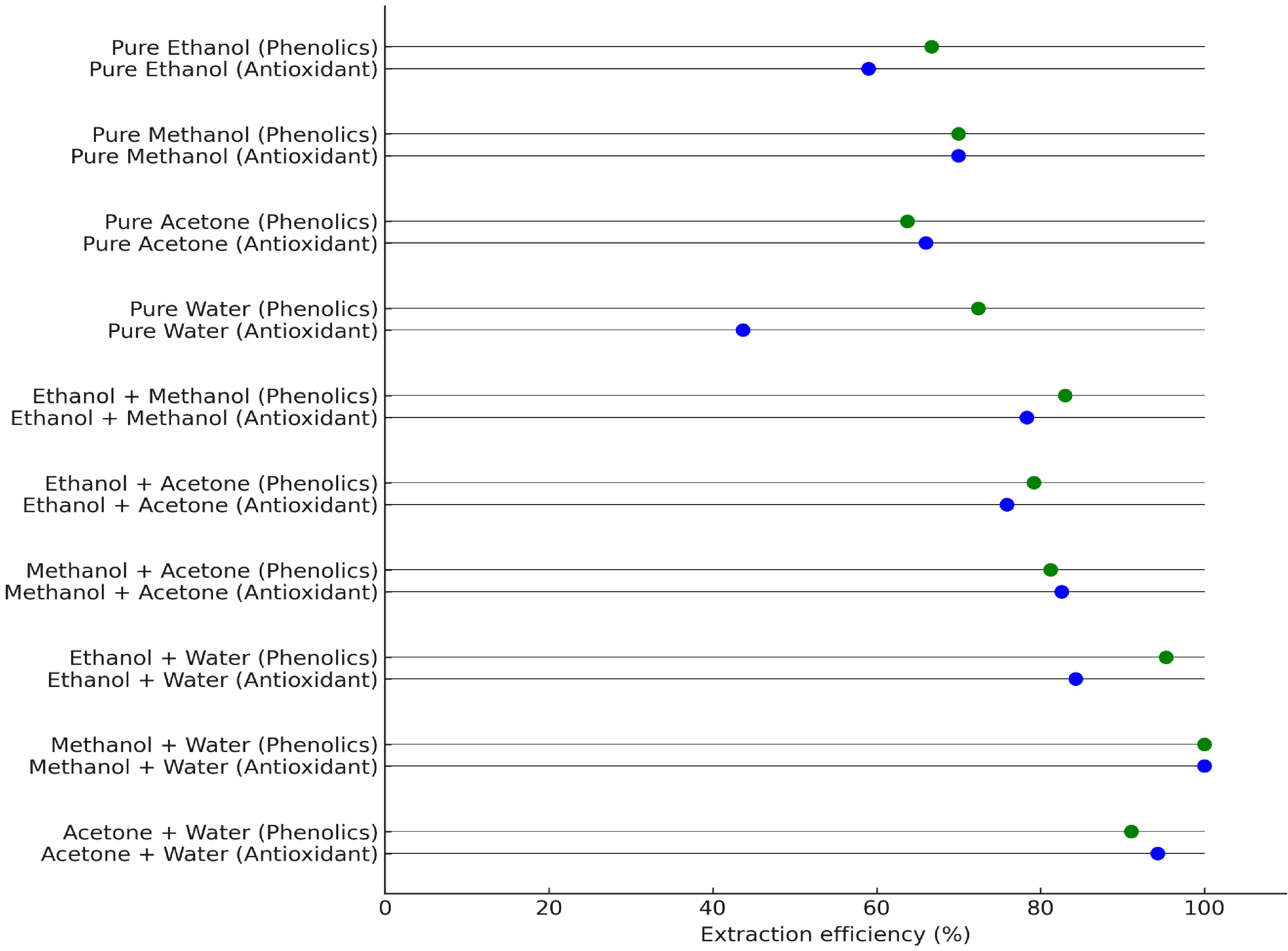
Figure 2.
Comparative efficiency scores of different solvent combinations for the extraction of phenolic and antioxidant compounds from pitaya, expressed as a percentage relative to the maximum observed efficiency.
This approach provided a practical and statistically robust method to optimize the extraction conditions without requiring exhaustive factorial designs.
To obtain the extracts used for determining bioactive compounds (antioxidants, betalains, and total phenolics), 2 g of the sample and 40 mL of the solvent were used, applying the simplex-centroid experimental design. In this study, solvents with high polarity (ethanol, methanol, and water) were selected to optimize the extraction of bioactive compounds. However, a solvent with intermediate polarity (acetone) was also used, as some bioactive compounds, such as flavonoids, which are present in significant amounts in pitaya, are more soluble in solvents of intermediate polarity, which may influence the extraction efficiency[1,23,24].
For satisfactory extraction, the use of solvent mixtures is recommended, which can be binary, ternary, or multicomponent, because in various extraction systems, the use of isolated solvents often results in unsatisfactory extractions[25]. Additionally, low-polarity solvents were not included, as they are typically employed for the extraction of nonpolar compounds such as lipids or waxes, which were beyond the scope of this study. Given that the main target compounds of pitaya are predominantly polar or semi-polar, medium- to high-polarity solvents were considered more appropriate and environmentally safer, particularly with the inclusion of ethanol and water.
In this context, the samples were extracted using 20 mL of a solvent system composed of 50% water and 50% of the designated organic solvent (Table 1), placed in an ultrasonic bath operating at room temperature (~25 °C) and a fixed frequency of 40 kHz for 30 min, and then processed by centrifugation for 15 min. The supernatant was filtered through quantitative filter paper (12.5 cm in diameter; 0.025 mm pore size). To ensure the maximum recovery of bioactive compounds, a second extraction was performed on the residual solid, following the same procedure and using 20 mL of the same solvent system. The extracts from both extractions were pooled and analyzed together to represent the overall extraction efficiency.
Table 1. Planning of mixtures for the extraction of bioactive compounds from pitaya.
F Acetone (%) Ethanol (%) Methanol (%) Water (%) F1 50 0 0 50 F2 0 50 0 50 F3 0 0 50 50 F4 25 25 0 50 F5 0 25 25 50 F6 25 0 25 50 F: Formulation. Antioxidant activity (ABTS+)
-
The ABTS+ assay demonstrates the sample's ability to reduce the 2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical. ABTS+ radical cations were produced by reacting a 7 mM ABTS stock solution with 145 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 hours before use. The ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. After adding 30 μL of the sample to 3 mL of the diluted ABTS+ solution, the absorbance was recorded 6 minutes after mixing. Ascorbic acid solutions (1–6 mg·mL−1) were used as the standard and positive control. The results were expressed as a percentage of reduction using the following equation:
$ {ABTS}^+\;Reduction\;{\text{%}}=\dfrac{Abs.control-Abs.sample\times 100}{Abs.control} $ (1) Where Abs.Control is the aAbsorbance of the initial ABTS radical solution without the sample and Abs.Sample is the absorbance of the reaction mixture after 6 min of reaction)[26].
Complementary antioxidant assays
-
Complementary antioxidant analyses were conducted, including total flavonoid content, determined by a spectrophotometric reaction with aluminum chloride and the phosphomolybdenum complex assay, based on the reduction of Mo(VI) to Mo(V). Additionally, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was tested, but due to strong spectral interference from betalain pigments, which compromised the reliability of the readings, these data were not included. Therefore, only the procedures and results related to flavonoids and phosphomolybdenum are presented in Supplementary Table S1.
Total phenolics
-
The total phenolic content was determined by the Fast Blue method described by Medina[27]. The reading was performed using a microplate reader (EZ Read 2000, Biochrom®) at 420 nm. The results were expressed in milligrams of gallic acid equivalents per 100 g of fresh weight of the sample (mg GAE 100 g−1 FM)
Betalains
-
The betalaine content was determined according to the method of Qingzhu et al.[28], with modifications by Zitha et al.[1]. The contents of betacyanin and betaxanthin were calculated using the following equations:
$ Betacyanin\; (mg\cdot100\; g^{-1}FM)=\dfrac{A_{538}\times DF\times MM\times V\times100}{\varepsilon\times P\times l} $ (2) $ Betaxanthin\; (mg\cdot100\; g^{-1}FM)=\dfrac{A_{483}\times DF\times MM\times V\times100}{\varepsilon\times P\times l} $ (3) where A538 is the absorbance for betacyanin at 538 nm, A483 is the absorbance for betaxanthins, DF is the dilution factor, MM is the molecular mass (550 g·mol−1 for betanin and 308 g·mol−1 for indicaxanthin), V is the volume of the pigment solution (mL),
$\varepsilon $ Statistical analysis
-
The extracts obtained from pitaya using different solvent formulations were evaluated with five biological replicates, each analyzed in triplicate for antioxidant activity assays, betalain levels (betacyanin and betaxanthin), and phenolic compounds. The data were analyzed using analysis of variance (ANOVA), followed by Tukey's post hoc test for mean comparisons at a 5% significance level (p < 0.05). A principal component analysis (PCA) with standardized data (z-score) was performed to evaluate the efficiency of the solvent formulations, using a biplot and 95% confidence ellipses to highlight clustering among formulations. All statistical analyses were performed using R version 4.5.0.
-
The use of solvents is essential in the extraction of bioactive compounds and is widely adopted by researchers. The combination of water with polar solvents such as methanol, ethanol, and acetone, among others, is frequently employed in the extraction of bioactive compounds from plants[24,29]. In this context, the present study focused on evaluating the efficiency of different solvent combinations in extracting bioactive compounds from various pitaya cultivars. It is important to highlight that the choice of 50% solvents significantly contributed to the sustainability of the process, as it reduced the use of large quantities of chemicals, minimizing environmental impacts and the risks of exposure to toxic compounds. This approach not only makes the extraction process safer but also more efficient, aligning with sustainable research and production practices[20].
Figure 3 shows the box plot, which evaluates the effect of different solvent combinations on the extraction of bioactive compounds (antioxidant activity, total phenolics, betacyanins, and betaxanthins) from the 'Boreal Red' pitaya cultivar.
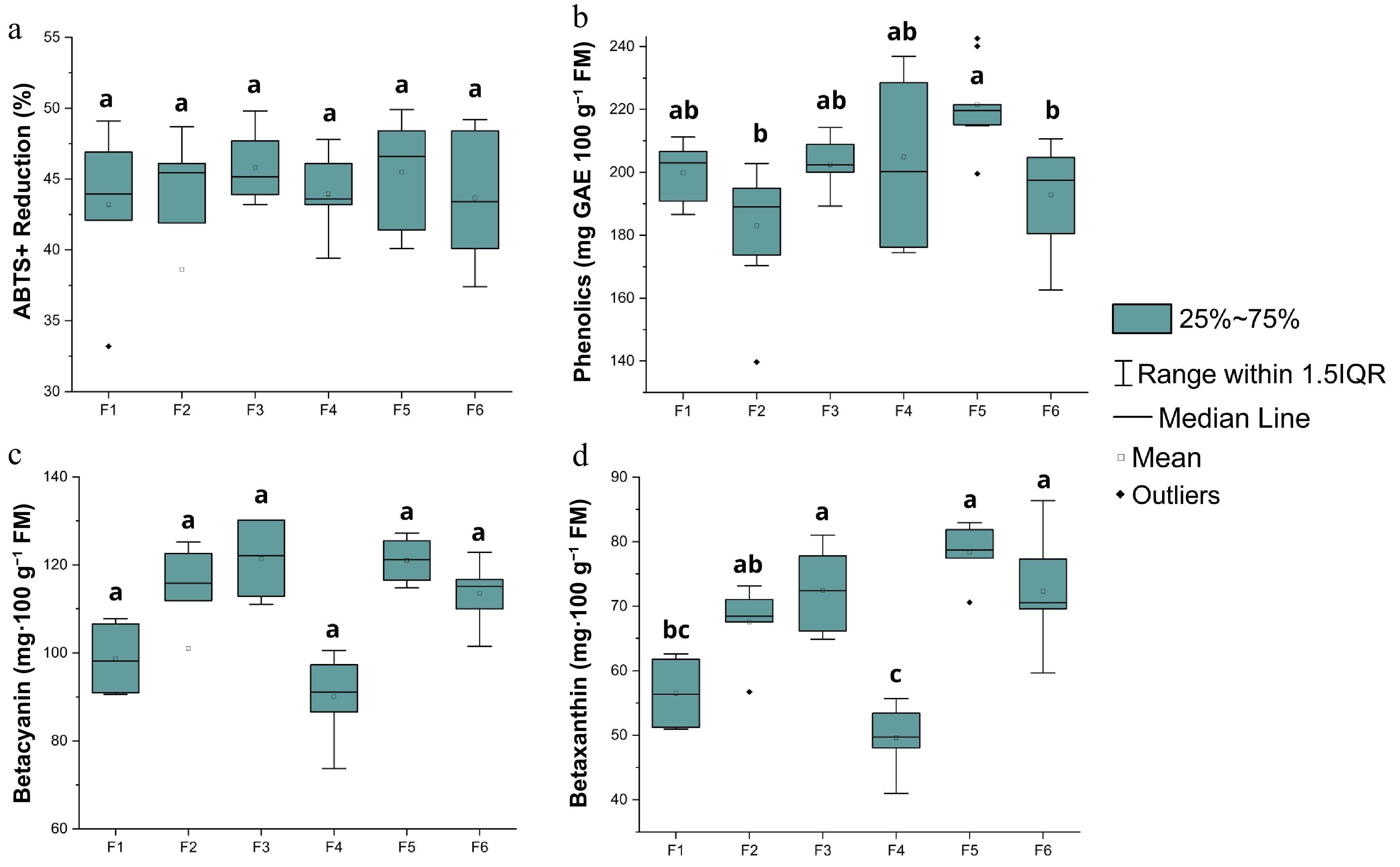
Figure 3.
(a) Antioxidant activity, (b) total phenolics, (c) betacyanin, and (d) betaxanthin of the 'Boreal Red' cultivar. Means with the same letters do not differ significantly according to Tukey's test at a 5% significance level.
The analysis of the ABTS+ assay results indicated that there were no statistically significant differences among the treatments (p > 0.05). However, formulations F3 (50% methanol and 50% water) and F5 (25% ethanol, 25% methanol, and 50% water) showed the highest averages (45.82% and 45.50%, respectively). These findings suggest that the combination of methanol with other solvents, such as ethanol or water, similarly optimized the extraction of antioxidant compounds. Methanol, due to its high polarity, is particularly effective in solubilizing phenolic compounds and flavonoids, which are substances commonly associated with antioxidant activity[30,31]. This combination enhanced the extraction of these compounds, promoting a higher antioxidant capacity in the extracts.
Among the tested formulations, F5 (25% ethanol, 25% methanol, and 50% water) exhibited the highest mean phenolic content (221.55 mg GAE 100 g−1 FM). Statistical analysis confirmed that F5 was significantly superior to formulations F2 and F6 (p < 0.05), and although not it was statistically different from F1, F3, and F4, it consistently presented numerically higher values across the replicates. This synergy can be explained by the complementarity between the solvents, promoting a more comprehensive extraction and allowing the solubilization of a greater diversity of phenolic compounds present in pitaya, resulting in higher extraction efficiency.
On the other hand, formulations F2 (50% ethanol and 50% water) and F6 (25% acetone, 25% methanol, and 50% water) showed the lowest averages (182.99 and 192.82 mg GAE 100 g−1 FM, respectively), indicating that the combinations of ethanol with water or the addition of acetone to the methanol + water mixture are less effective in extracting phenolic compounds. This result can be attributed to the lower polarity of ethanol and acetone compared with methanol, which reduces their ability to solubilize high-polarity phenolic compounds. Higher-polarity solvents generally show greater efficiency in extracting polyphenols due to the high solubility of these compounds in highly polar media[32].
Furthermore, the inclusion of water in the formulations plays a crucial role in creating a polar medium, significantly enhancing the efficiency of phenolic compound extraction[32−35]. The synergy between water and organic solvents facilitates the expansion of the plant matrix, increasing the contact surface area between the fruit pulp and the solvent. This structural expansion optimizes the extraction process, allowing for greater recovery of bioactive compounds[36].
The betacyanins and betaxanthins belong to the larger group of betalains, the predominant pigments responsible for the characteristic coloration of pitaya. Betacyanins are associated with red to purple hues, while betaxanthins impart shades ranging from yellow to orange[37].
The results obtained for betacyanins revealed a clear predominance of the formulations containing methanol. The highest average concentrations were observed for formulations F3 (50% methanol and 50% water) and F5 (25% ethanol, 25% methanol, and 50% water), with values of 121.41 and 121.05 mg of betacyanin per 100 g of fresh matter (FM), respectively. In contrast, the formulations containing acetone (F1 and F4) exhibited the lowest averages (98.69 and 90.04 mg of betacyanin 100 g−1 FM), indicating that acetone is less efficient in extracting this type of pigment. This is likely due to its lower polarity and limited ability to dissolve highly polar compounds, such as betacyanins, which have high polarity and a hydrophilic structure, favoring solubility in more polar solvents[38].
Regarding betaxanthins, which are compounds derived from the conjugation of acids with amino acids or amines, the results also showed significant statistical differences. The formulation F5 (25% ethanol, 25% methanol, and 50% water) had the highest betaxanthin content, with 78.39 mg·100 g−1 FW, followed by F3 (50% methanol and 50% water) and F6 (25% acetone, 25% methanol, and 50% water), which also showed high values (72.45 and 72.27 mg·100 g−1 FW, respectively), belonging to the same statistical group. These data reinforce the superiority of methanol, either alone or in combination with other solvents, in extracting betaxanthins. On the other hand, the formulations F4 and F1 showed the lowest averages, reflecting the lower efficiency of these solvents in extracting betaxanthins. This can be attributed to the intermediate polarity of acetone, which, although polar, is considered an aprotic solvent, meaning it does not have hydrogen atoms attached to the electronegative atoms. As a result, it has less ability to interact with highly polar compounds, such as betaxanthins, which require a more polar environment for efficient dissolution[39,40].
'Chinesa' cultivar
-
Figure 4 shows the box plot evaluating the effect of different solvent combinations on the extraction of bioactive compounds (antioxidant activity, total phenolics, betacyanins, and betaxanthins) from the 'Chinesa' pitaya cultivar.
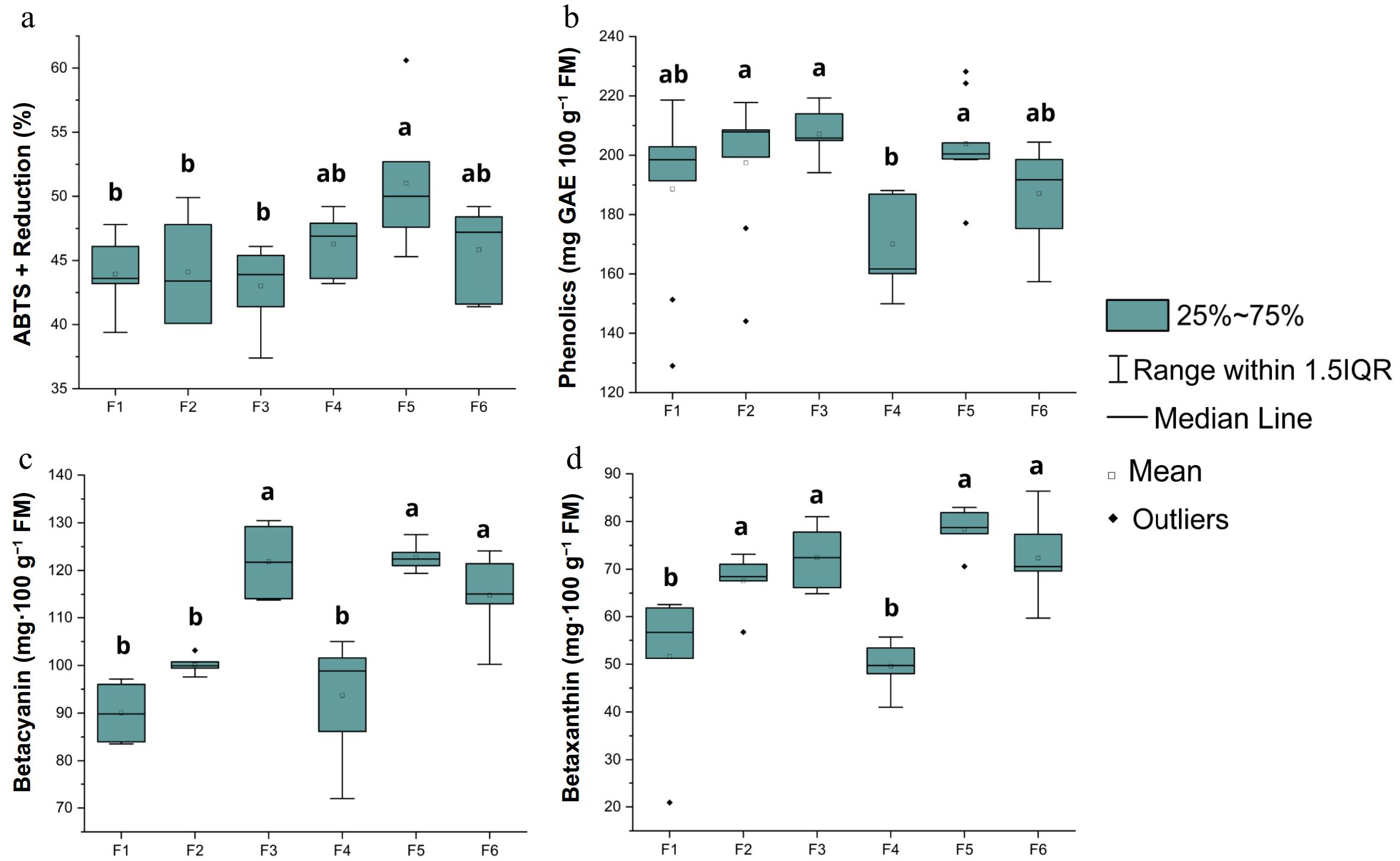
Figure 4.
(a) Antioxidant activity, (b) total phenolics, (c) betacyanin, and (d) betaxanthin of the 'Chinesa' cultivar. Means with the same letters do not differ significantly according to Tukey's test at a 5% significance level.
Among the reduction percentages achieved through the scavenging of free radicals by the antioxidants[41], as shown in the contour plot (Fig. 4), the ternary formulation (F5) exhibited the highest reduction at 51.03%, followed by F4 (46.28%) and F6 (45.83%). The highest antioxidant activity observed in F5 was obtained with the combination of methanol, ethanol, and water as solvents. These solvents are effective in extracting antioxidant compounds such as phenols, flavonoids, and anthocyanins, which are well known for their ability to neutralize free radicals. This result is attributed to the synergy of the solvents, facilitated by their polarities, where water aids in the extraction of hydrophilic compounds[25,42], while the alcohols contribute to the extraction of a broad range of phenolic constituents[43]. Similar findings in the literature demonstrate that solvent mixtures exhibit a greater capacity for extracting antioxidants compared with individual solvents[40,44].
The formulations F3 (207.17 mg GAE 100 g−1 FM), F5 (203.91 mg GAE 100 g−1 FM), and F2 (197.45 mg GAE 100 g−1 FM) exhibited the highest concentrations of phenolic compounds, with no statistically significant differences among them. This highlights the effectiveness of the methanol and ethanol combination in extracting these compounds. In contrast, the formulation F4 showed the lowest extraction yield, with 170.14 mg GAE 100 g−1 FM, suggesting that the acetone present in this mixture may not be as efficient in extracting phenolic compounds.
Similar results were observed in another study, where the total phenolic content in red-fleshed pitaya (Hylocereus polyrhizus) ranged from 130.91 to 249.17 mg GAE 100 g−1 FM during the anthesis period (from Day 28 to Day 34), using 50% methanol[1]. The phenolic compound content is generally higher when water is present, as its combination with organic solvents creates a polar medium that facilitates the extraction of these bioactive compounds. Furthermore, the presence of water can promote the expansion of the raw material through absorption, increasing the surface area in contact with the solvent and consequently improving the extraction yield[33,34,45].
The different combinations of solvents significantly influenced the extraction of betalains from the 'Chinesa' cultivar of pitaya. Regarding betacyanin content, the formulations F5 (122.77 mg·100 g−1 FM), F3 (121.85 mg·100 g−1 FM), and F6 (114.81 mg·100 g−1 FM) exhibited the highest concentrations, while F2 (100.12 mg·100 g−1 FM), F1 (90.05 mg·100 g−1 FM), and F4 (93.72 mg·100 g−1 FM) showed the lowest values.
For betaxanthin content, formulations F5 (78.39 mg·100 g−1 FM), F3 (72.45 mg·100 g−1 FM), F6 (72.34 mg·100 g−1 FM), and F2 (67.56 mg·100 g−1 FM) had the highest pigment concentrations, whereas F4 (49.59 mg·100 g−1 FM) and F1 (51.65 mg·100 g−1 FM) demonstrated the lowest values. These findings indicate that the solvent composition plays a critical role in the efficiency of betalain extraction, with polar solvents, particularly in ternary and binary combinations, being more effective in enhancing the solubility and recovery of these bioactive pigments.
Similar to the present study, the betacyanin content in the extract of red-fleshed pitaya (Hylocereus polyrhizus) during the post-anthesis period (between Days 34 and 42) ranged from 152.40 to 250.19 mg·100 g−1 FM. In contrast, the observed values for betaxanthin were lower, ranging from 41.65 to 59.87 mg·100 g−1 FM when using 80% methanol as the solvent[1]. These divergences can be attributed to differences in the solvents used, the cultivars analyzed, and the specific characteristics of the betalain compounds. This reinforces the notion that, so far, there is no ideal solvent that can be generalized for the efficient extraction of betalains across all cultivars.
'Roxa do Pará' cultivar
-
Figure 5 shows the box plot evaluating the effect of different solvent combinations on the extraction of bioactive compounds (antioxidant activity, total phenolics, betacyanins, and betaxanthins) from the 'Roxa do Pará' pitaya cultivar.
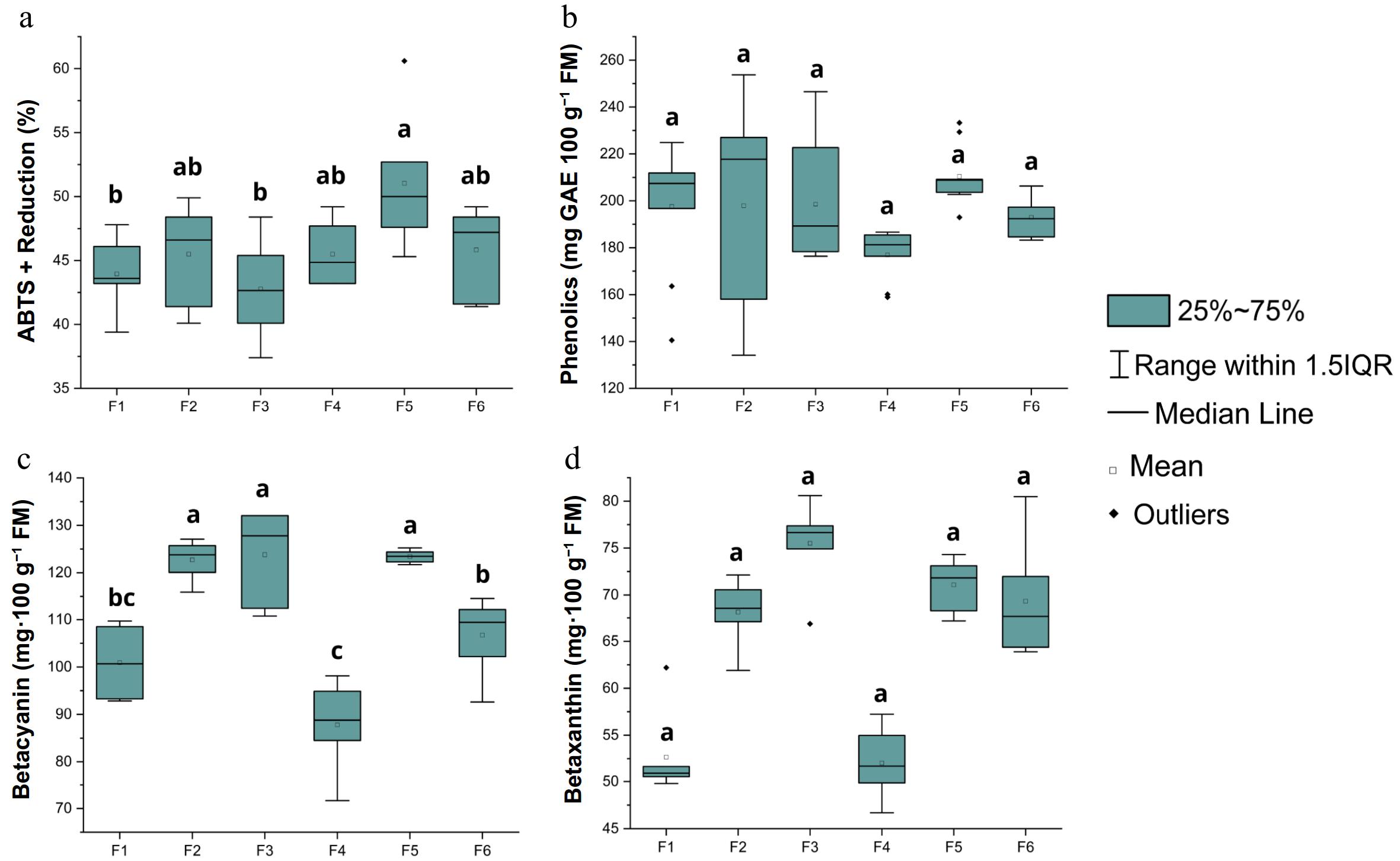
Figure 5.
(a) Antioxidant activity, (b) total phenolics, (c) betacyanin, and (d) betaxanthin of the 'Roxa do Pará' cultivar. Means with the same letters do not differ significantly according to Tukey's test at a 5% significance level.
The antioxidant activity of the 'Roxa do Pará' pitaya cultivar, assessed by the reduction of the ABTS radical, showed significant variations among the formulations. Formulation F5 was the most efficient, with a reduction of 51.03%, followed by formulations F2, F4, and F6, which achieved reductions of 45.50%, 45.50%, and 45.83%, respectively. These results indicate that the combination of solvents in the formulations directly impacts the ability to capture free radicals. As observed in other cultivars, formulation F5, comprising methanol, ethanol, and water, demonstrated a consistently high extraction capacity, particularly for antioxidant compounds. This performance may be attributed to the synergistic action between solvents of different polarities, which enhances the solubilization of structurally diverse bioactive compounds. Although not always statistically superior to all formulations, F5 repeatedly yielded among the highest values across multiple parameters.
These findings corroborate the literature, which suggests that combined solvents are more effective in extracting antioxidant compounds than individual solvents. Several authors have reported similar results, observing higher antioxidant activity in formulations using combined solvents, especially when water is present in the mixture, increasing the extraction potential of bioactive compounds[40,44].
The results obtained for the phenolic compound content indicated that all formulations presented considerable concentrations of phenolics (p > 0.05), with formulation F5 (210.42 mg GAE 100 g−1 FM) achieving the highest yield of phenolic compounds. The absence of statistical differences between formulations F1, F2, F5, and F6 suggests that the combination of solvents, even with different proportions of methanol, ethanol, and water, may have a positive impact on the extraction of phenolic compounds. Formulation F5, which includes methanol, ethanol, and water, achieved the highest yield of phenolic compounds, which can be explained by the combined efficacy of these solvents in extracting both water-soluble and liposoluble compounds. As previously mentioned, the presence of water facilitates the extraction of polar compounds, while the alcohols help extract a broader range of phenolics[25,43].
Other evidence in the literature suggests that polar mixtures (water/acetone 50%) were effective in extracting phenolics from date palm fruits (Phoenix dactylifera L.)[46]. Similarly, for strawberry extracts[42], it was reported that the highest amount of total phenolic compounds was obtained with acetone/water extraction solutions at ratios of 50/50 (v/v) and 70/30 (v/v).
The results for the betacyanin concentrations in the 'Roxa do Pará' pitaya cultivar showed significant variations among the solvent formulations. The F5 formulation (123.42 mg·100 g−1 FM) had the highest betacyanin concentration, followed by the F3 formulation (123.80 mg·100 g−1 FM) and F2 (122.72 mg·100 g−1 FM), with very close values. In contrast, the F4 formulation had the lowest betacyanin yield (87.79 mg·100 g−1 FM), suggesting that the presence of acetone may not be as effective in extracting this specific pigment, possibly due to its polarity, which may not be ideal for extracting compounds like betacyanin.
On the other hand, no statistically significant differences were observed in the betaxanthin content among the formulations (p = 0.341), in contrast to the results obtained for the 'Boreal' (Fig. 3) and 'Chinesa' (Fig. 4) cultivars, which showed clear statistical variation among the treatments. In 'Roxa do Pará', betaxanthin content ranged from 52.66 to 75.51 mg·100 g−1 FM, with the F3 formulation showing the highest content, followed by F5 and F6. The other formulations had values below 68.12 mg·100 g−1 FM, indicating that, although the type of solvent impacts betacyanin concentrations, its effect on betaxanthin extraction is less pronounced. This apparent homogeneity in betaxanthin content may also be influenced by the genetic characteristics of the cultivar evaluated. It is noteworthy that 'Roxa do Pará' belongs to a different species (Selenicereus costaricensis) from the other cultivars assessed (S. undatus), which may contribute to the more stable profile of certain bioactive compounds such as betaxanthins. Further studies comparing species-specific metabolic pathways could help clarify these observations.
Figure 6 presents the PCA performed to evaluate the overall efficiency of the solvent formulations in extracting bioactive compounds from pitaya. The PCA provided a comprehensive multivariate assessment, revealing patterns of association between formulations and the response variables.
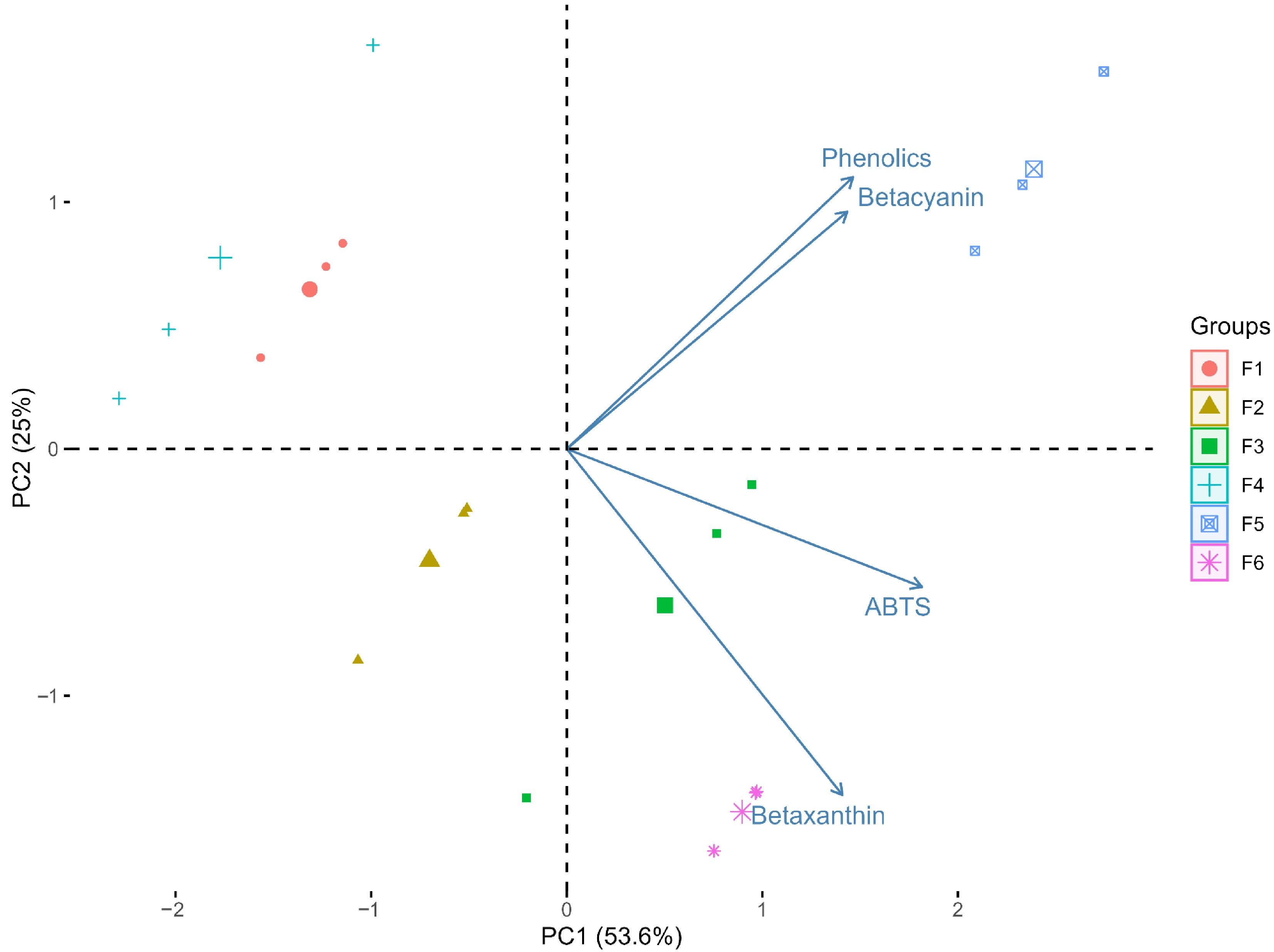
Figure 6.
PCA of solvent formulations for the extraction of bioactive compounds of pitaya. The biplot shows extraction efficiency and clustering among the formulations.
PCA explained 78.6% of the total variance in the first two components (Principal Component (PC)1: 53.6%; PC2: 25%), indicating that the multivariate patterns reliably summarize the relationships between variables and formulations. Formulations F5, F3, and F2, combining water with organic solvents (methanol and ethanol), stood out for their higher extraction efficiency of betacyanin and betaxanthin. This highlights the essential role of water as a component that modulates polarity and facilitates the solubilization of bioactive compounds. Conversely, formulations with a higher proportion of acetone or without water (F1) exhibited lower extraction efficiency. The multivariate approach also underscores the importance of solvent synergy, with different polarities improving the extraction profile across multiple compounds.
Furthermore, the partial substitution of solvents with water represents a significant advancement towards green chemistry, promoting greater sustainability without compromising analytical efficiency[47]. Pitaya, highly appreciated worldwide for its aesthetic appeal and nutritional value, is often considered a superfruit. Consequently, it remains a constant focus regarding the identification and quantification of its bioactive constituents, given its complex functional composition. The multivariate approach adopted in this study is therefore essential for advancing the understanding of extraction mechanisms and enhancing the scientific valorization of these fruits[48].
-
In conclusion, the results of this study highlight the importance of solvent combinations in the extraction of bioactive compounds from pitaya cultivars. Formulations containing methanol, ethanol, and water, particularly F5 (25% ethanol, 25% methanol, and 50% water), proved to be the most efficient for extracting antioxidant compounds, phenolics, and betalains, with high concentrations of betacyanins and betaxanthins. The synergy among these solvents, due to their complementary polarities, enhanced the extraction process, allowing for the solubilization of a broader range of bioactive compounds. Conversely, acetone-based formulations were less effective, particularly in extracting betacyanin, suggesting that its polarity may not be optimal for certain pigments. Additionally, the use of water in the solvent mixtures contributed to the sustainability of the extraction process, reinforcing the potential for developing environmentally friendly methods for bioactive compound determination in fruits like pitaya. Water, as a sustainable solvent, plays a key role in reducing the overall toxicity of the extraction process by decreasing the reliance on toxic solvents. Thus, the solvent combinations tested, particularly F5, provide a balanced approach, combining high efficiency in the extraction of bioactive compounds with a commitment to environmentally responsible practices. This integration aligns with the principles of green chemistry, promoting a safer and more sustainable methodology for the extraction of bioactive compounds. Overall, the findings emphasize the significance of solvent selection in optimizing the extraction of bioactive compounds, with implications for both efficiency and sustainability in research and industrial applications.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001, the Brazilian National Council for Scientific and Technological Development (CNPq), and the Research Support Foundation of the State of Minas Gerais (FAPEMIG).
-
The authors confirm contributions to the paper as follows: study conception and design: Da Silva LGM, Carvalho EEN, Vilas Boas EVDB; data collection: Da Silva LGM, Da Costa CAR, de Abreu DJM; analysis and interpretation of results: Da Silva LGM, Da Costa CAR, Batista GA, Amorim KA, de Abreu DJM, Carvalho EEN; supervision: de Barros Vilas Boas EV, Pio LAS, Carvalho EEN; draft manuscript preparation: Da Silva LGM, Batista GA, Pio LAS, Amorim KA, Carvalho EEN. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Supplementary analyses of flavonoid content and phosphomolybdenum complex.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
da Silva LGM, da Costa CAR, Batista GA, Amorim KA, de Abreu DJM, et al. 2025. Synergy between solvent polarity and composition for efficient extraction of bioactive compounds from pitaya. Food Materials Research 5: e012 doi: 10.48130/fmr-0025-0012
Synergy between solvent polarity and composition for efficient extraction of bioactive compounds from pitaya
- Received: 26 February 2025
- Revised: 27 May 2025
- Accepted: 30 June 2025
- Published online: 28 July 2025
Abstract: The polarity of a solvent and the characteristics of bioactive compounds are crucial factors that influence the efficiency of extraction and the composition of extracts. In this context, the use of solvent mixtures can be an effective alternative to optimize the extraction process. This study aimed to optimize the extraction of bioactive compounds (antioxidants, phenolics, and betalains) from pitaya cultivars ('Boreal Red', 'Chinese', and 'Roxa do Pará') using combinations of extracting solvents with water, namely acetone, methanol, and ethanol, both isolated and combined. To optimize, binary and ternary mixtures were tested, resulting in six formulations. The extracts were prepared with the extractor, followed by an ultrasonic bath, centrifugation, and filtration. A second extraction was performed using the filtrate supernatant. The use of solvents partly combined with water proved efficient in extracting compounds of agro-food interest, aligning with green chemistry principles and environmental protection. The formulations containing methanol, ethanol, and water, especially F5 (25% ethanol, 25% methanol, and 50% water), proved to be the most efficient for extracting antioxidant compounds, phenolics, and betalains, with high concentrations of betacyanins and betaxanthins. In all cultivars, F5 outperformed other formulations, with increases of up to 25.8% in antioxidant activity, 23.5% in total phenolics, 22.7% in betacyanins, and 27.0% in betaxanthins compared with the least effective solvents. The synergy between these solvents, due to their complementary polarities, enhanced the extraction process.
-
Key words:
- Green chemistry /
- Antioxidants and betalains /
- Pitaya cultivars













