-
Gastric cancer (GC) remains a leading cause of cancer-related mortality worldwide. According to the 2022 Global Cancer Statistics (released by IARC in 2024), GC accounted for 4.9% (968,000 of 19.96 million) of new cancer cases, and 6.8% (660,000 of 9.74 million) of cancer deaths globally[1]. The human epidermal growth factor receptor 2 (HER2/ERBB2), encoded by the ERBB2 gene (chromosome 17q12), activates the PI3K/AKT and RAS/MAPK signaling pathways to regulate cellular proliferation, differentiation, and survival. In gastric adenocarcinoma, ERBB2 amplification represents a therapeutic target, with anti-ERBB2 agents demonstrating survival benefits in amplification-positive tumors[2]. Large-scale studies (n = 4,920)[3] report an overall ERBB2 amplification rate of approximately 14.2% in gastric adenocarcinoma. Notably lower in Asian vs Western populations due to histological and methodological variations.
DNA mismatch repair (MMR) deficiency (dMMR) impairs the correction of DNA replication errors, leading to microsatellite instability (MSI)[4]. The prevalence of dMMR/MSI in GC ranges from 5.6% to 23.5% (median: 13.7% in Asians), and is associated with proximal tumor location, expansive growth patterns, and high tumor-infiltrating lymphocyte (TIL) density[5−7]. Despite frequent poor differentiation, dMMR/MSI tumors demonstrate a favorable prognosis and enhanced response to immune checkpoint inhibitors.
This study investigates the association between ERBB2 amplification and dMMR status in gastric adenocarcinoma (GAC), and evaluates their joint impact on clinicopathological features (differentiation, PD-L1 expression), and overall survival to guide precision therapeutic strategies.
-
This retrospective study screened 1,104 consecutive patients with newly diagnosed gastric adenocarcinoma who underwent ERBB2 testing at the First People's Hospital of Changzhou (January 2018–June 2022). After exclusions, 176 patients met the following criteria:
Inclusion criteria:
1. Pathologically confirmed gastric adenocarcinoma;
2. No prior anti-tumor therapy at initial diagnosis;
3. Complete clinicopathological and follow-up data;
4. Signed informed consent.
Exclusion criteria:
1. Inadequate specimen quality;
2. Concurrent malignancies or hereditary tumor syndromes.
All specimens underwent 10% neutral-buffered formalin fixation (24–48 h) and paraffin embedding. Tumor staging adhered to the 8th UICC/AJCC TNM classification determined via independent review by two senior pathologists blinded to molecular data. Clinicopathological variables were retrieved from electronic medical records. Survival data were updated via structured telephone interviews through June 2024. Ethical approval was obtained from the Ethics Committee of the First People's Hospital of Changzhou [Approval No. 2023 (教) CL087-01].
Molecular analyses
ERBB2 status assessment
-
ERBB2 protein expression was initially evaluated by immunohistochemical (IHC). Fluorescence in situ hybridization (FISH) was subsequently performed to confirm ERBB2 gene amplification. ERBB2 status was classified according to the 2018 ASCO/CAP guidelines. ERBB2-positive: Gene amplification (ERBB2/CEP17 ratio ≥ 2.0) and ERBB2-negative group: No gene amplification (ERBB2/CEP17 ratio < 2.0).
MMR status assessment
-
Mismatch repair (MMR) protein expression (MLH1, MSH2, MSH6, PMS2) was evaluated by immunohistochemistry (IHC) using validated antibodies, samples were classified as: pMMR/MSS: Intact nuclear expression in tumor cells. dMMR/MSI: Loss of nuclear expression in ≥ 1 protein (with internal controls).
PD-L1 assessment
-
PD-L1 expression was quantified using the PD-L1 IHC 22C3 pharmDx assay (Dako/Agilent Technologies, Santa Clara, CA, USA; batch number 11756940) on the Dako Autostainer Link 48 platform. The Combined Positive Score (CPS) was calculated as: CPS = (Number of PD-L1 cells [tumor cells, lymphocytes, macrophages]/Total viable tumor cells) × 100. Samples were stratified as: High-expression: CPS ≥ 5, Low-expression: CPS < 5. (For reagent details and representative results see Table 1 and Fig. 1)
Table 1. Antibodies and reagents used in the study.
Target protein Company Product name Item No. Batch No. Clone No. Antibody type PMS2 Beijing Zhongshan Jinqiao Biotechnology PMS2 protein ZM-0407 24090414 OTI4B2 Mouse monoclonal MSH2 Beijing Zhongshan Jinqiao Biotechnology MSH2 protein ZA-0702 25012033 OTIR1B12 Rabbit monoclonal MSH6 Beijing Zhongshan Jinqiao Biotechnology MSH6 protein ZM-0367 24111703 UMAB258 Mouse monoclonal MLH1 Beijing Zhongshan Jinqiao Biotechnology MLH1 protein ZM-0152 24112706 OTI4H4 Mouse monoclonal HER-2 Roche Diagnostics GmbH HER-2/NEU antibody reagent (IHC method) − (10) M22565 − − PD-L1 Dako Monoclonal Mouse Anti-PD-L1, Clone 22C3 − 11756940 22C3 Mouse monoclonal 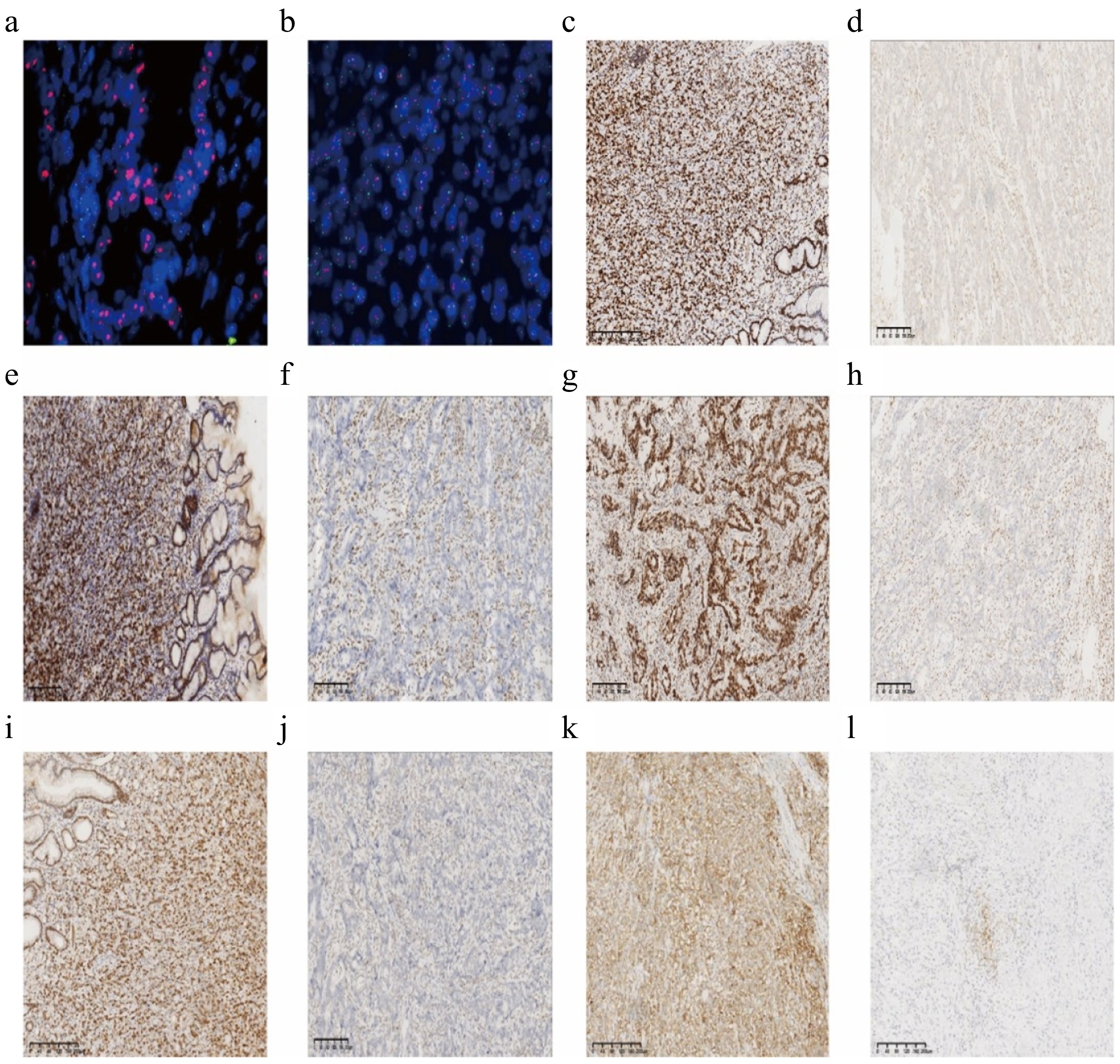
Figure 1.
Representative immunohistochemical staining in gastric adenocarcinoma. (a) ERBB2 amplification (HER-2 positive); (b) ERBB2 amplification was not obvious (HER-2 negative); (c) expression of MLH1 protein; (d) deletion of MLH1 protein; (e) expression of MSH2 protein; (f) deletion of MSH2 protein; (g) expression of MSH6 protein; (h) deletion of MSH6 protein; (i) expression of PMS2 protein; (j) deletion of PMS2 protein; (k) positive PD-L1 (CPS ≥ 5); (l) PD-L1 negative (CPS < 5). Scale = 200 μm.
Survival analysis
-
Disease-free survival (DFS): Time from curative-intent resection to recurrence, metastasis, or last follow-up (censoring: non-cancer deaths/loss to follow-up). Overall survival (OS): Time from diagnosis confirmation to all-cause death or last follow-up (censored: alive at June 2024).
Statistical analysis
-
Analyses used SPSS 22.0 (IBM Corp.): Continuous variables: Independent t-tests (normally distributed). Categorical variables: χ2 or Fisher's exact tests (expected counts < 5). Survival curves: Kaplan-Meier method with log-rank testing. Statistical significance: p < 0.05 (two-tailed).
-
Among 176 patients, 26 (14.8%) were ERBB2-positive. Key findings include:
Sex distribution: ERBB2-positive group: 84.62% male vs 15.38% female. ERBB2-negative: 71.33% male (p = 0.158).
Age: ≥ 65 years: 65.38% (ERBB2+) vs 56.00% (ERBB2−) (p = 0.372).
Tumor differentiation: Significant association observed (p = 0.047): ERBB2+: Moderate differentiation (50.00%), poor (23.08%), unspecified (26.92%). ERBB2−: Moderate differentiation (36.00%), poor (48.67%) suggesting a potential association between tumor differentiation and ERBB2 status.
Surgical management: Primary surgery: 80.77% (ERBB2+) vs 90.00% (ERBB2−) (p = 0.270).
TNM staging: T-stage: T2/T3 predominance (92.31% ERBB2+) vs 80.00% (ERBB2−) (p = 0.622). M1 disease: 19.23% (ERBB2+) vs 8.67% (ERBB2−) (p = 0.151). Full data is provided in Table 2.
Table 2. Relationship between ERBB2 gene amplification and clinicopathological features.
Clinicopathological features ERBB2(−)
(n = 150)ERBB2(+)
(n = 26)χ2 p-value Gender 1.997 0.158 Male 107 (71.33%) 22 (84.62%) Female 43 (28.67%) 4 (15.38%) Age 0.798 0.372 < 65 66 (44.00%) 9 (34.62%) ≥ 65 84 (56.00%) 17 (65.38%) Differentiation degree 8.935 0.047 High differentiated 3 (2.00%) 0 (0%) Moderately differentiated 54 (36.00%) 13 (50.00%) Poorly differentiated 73 (48.67%) 6 (23.08%) Signet ring cell 4 (2.67%) 0 (0%) NA 16 (10.67%) 7 (26.92%) Surgery 2.618 0.270 Initial surgery 135 (90.00%) 21 (80.77%) Neoadjuvant post-surgery 5 (3.33%) 2 (7.69%) None 10 (6.67%) 3 (11.54%) T stage 1.719 0.622 T1 14 (9.33%) 1 (3.85%) T2 39 (26.00%) 8 (30.77%) T3 81 (54.00%) 16 (61.54%) T4 16 (10.67%) 1 (3.85%) N stage 1.881 0.610 N0 52 (34.67%) 7 (26.92) N1 24 (16.00%) 6 (23.08%) N2 36 (24.00%) 8 (30.77%) N3 38 (25.33%) 5 (19.23%) M stage 0.151* M0 137 (91.33%) 21 (80.77%) M1 13 (8.67%) 5 (19.23%) * Fisher's exact test was used for M stage analysis. Association between dMMR status and clinicopathological features
-
Among 22 dMMR cases (12.5%):
Sex distribution: Strong female predominance in dMMR (59.09% female vs pMMR: 22.08%; p < 0.001).
Tumor differentiation: Moderate differentiation in dMMR (63.64% vs pMMR: 34.42%; p = 0.011).
T-stage: Trend toward earlier T-stage in dMMR (T3: 45.45% vs pMMR: 56.49%; p = 0.069). No significant differences in nodal status (p = 0.208) or metastasis (p = 1.000). Full data is provided in Table 3.
Table 3. Relationship between mismatch repair (MMR) gene status and clinicopathological features.
Clinicopathological features pMMR
(n = 154)dMMR
(n = 22)χ2 p-value Gender 13.473 <0.001 Male 120 (77.92%) 9 (40.91%) Female 34 (22.08%) 13 (59.09%) Age 0.083 0.773 < 65 65 (42.21%) 10 (45.45%) ≥ 65 89 (57.79%) 12 (54.55%) Differentiation degree 11.385 0.011 High differentiated 2 (1.30%) 1 (4.55%) Moderately differentiated 53 (34.42%) 14 (63.64%) Poorly differentiated 75 (48.70%) 4 (18.18%) Signet ring cell 3 (1.95%) 1 (4.55%) NA 21 (13.64%) 2 (9.09%) Surgery 0.535 0.861 Initial surgery 135 (87.66%) 21 (95.45%) Neoadjuvant post-surgery 7 (4.55%) 0 (0%) None 12 (7.79%) 1 (4.55%) T stage 6.253 0.069 T1 11 (7.14%) 4 (18.18%) T2 39 (25.32%) 8 (36.36%) T3 87 (56.49%) 10 (45.45%) T4 17 (11.04%) 0 (0%) N stage 4.507 0.208 N0 47 (30.52%) 12 (54.54%) N1 27 (17.53%) 3 (13.64%) N2 40 (25.97%) 4 (18.18%) N3 40 (25.97%) 3 (13.64%) M stage 1.000* M0 138 (89.61%) 20 (90.91%) M1 16 (10.39%) 2 (9.09%) * Fisher's exact test was used for M stage analysis. Association between ERBB2 amplification and dMMR status
-
No significant correlation was observed between ERBB2 amplification and dMMR status: ERBB2-negative cohort: pMMR 86.00% vs dMMR 14.00%, ERBB2-positive cohort: pMMR 96.15% vs dMMR 3.85% (p = 0.206) (Table 4).
Table 4. Association between ERBB2 expression and mismatch repair status.
Clinicopathological
featuresERBB2(−)
(n = 150)ERBB2(+)
(n = 26)χ2 p-value Mismatch repair status 0.206* pMMR 129 (86.00%) 25 (96.15%) dMMR 21 (14.00%) 1 (3.85%) * Fisher's exact test was used for statistical analysis. Relationships with PD-L1 expression
-
ERBB2 status: PD-L1 low (CPS < 5): 83.33% ERBB2-negative vs 16.67% ERBB2-positive, PD-L1 high (CPS ≥ 5): 86.73% ERBB2-negative vs 13.27% ERBB2-positive (p = 0.528).
dMMR status: PD-L1 low: 96.15% pMMR vs 3.85% dMMR, PD-L1 high: 80.61% pMMR vs 19.39% dMMR (p = 0.002).
Survival outcomes
-
The follow-up duration for 176 patients ranged from 1 to 97 months, with a median of 37.22 months. Five patients were lost to follow-up, yielding a loss rate of 2.84%. A total of 57 patients died, with a mortality rate of 32.39%.
ERBB2 status: No significant DFS difference (p = 0.812):
ERBB2-negative: 48 patients died, with a median DFS 40.312 ± 17.40 months,
ERBB2-positive: nine patients died, with a median DFS 38.000 ± 13.73 months.
MMR status: No significant DFS difference (p = 0.926):
pMMR group: 50 patients died, with a median DFS 40.67 ± 14.36 months,
dMMR group: seven patients died, with a median DFS 40.18 ± 14.49 months.
PD-L1 expression: Significantly improved DFS in PD-L1-high group (p = 0.015):
CPS < 5: 32 patients died, with a median DFS 41.08 ± 17.27 months,
CPS ≥ 5: 25 patients died, with a median DFS 40.31 ± 17.40 months.
On the surface, the average survival time seems to favor the PD-L1 negative group numerically, but the survival curve shows that the positive group is significantly superior. Patients with positive PD-L1 expression are more sensitive to immunotherapy and are more likely to be alive at the end of the study. Although these patients are included in the 'denominator', their survival time is censored (only recorded up to the last follow-up time), resulting in an underestimation of their actual survival contribution. In this case, the conclusions related to the survival curve should be given attention (Fig. 2, Table 5).
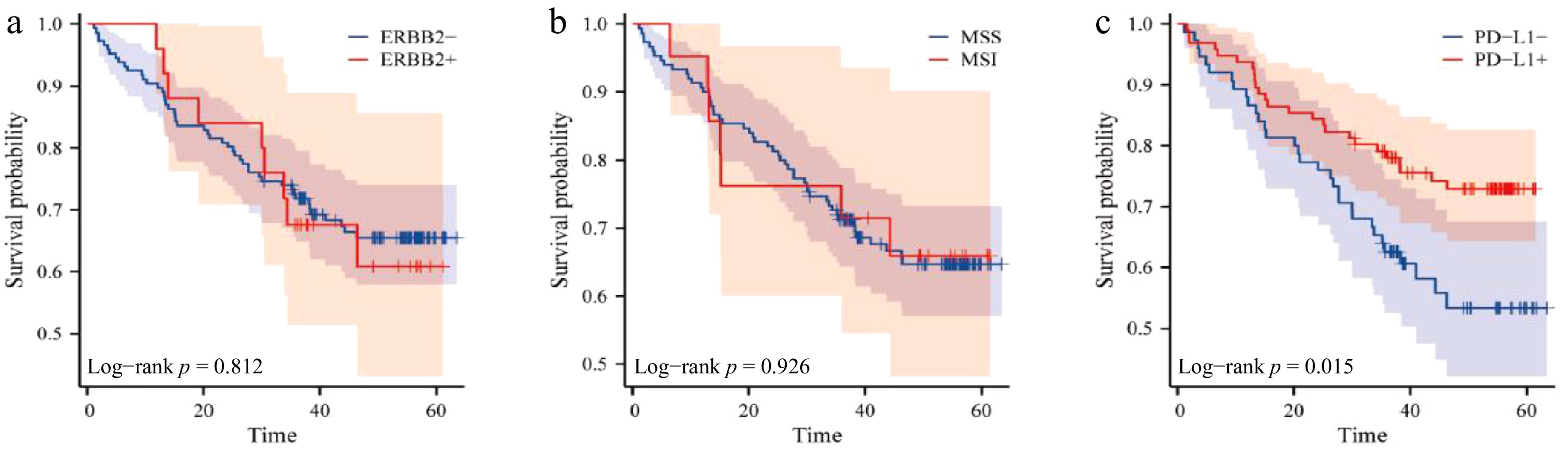
Figure 2.
The relationship between ERBB2, microsatellite status, PD-L1 expression, and prognosis of patients. (a) Survival curve of ERBB2 expression and prognosis; (b) survival curve of microsatellite status and prognosis; (c) survival curve of PD-L1 expression and prognosis.
Table 5. Association of ERBB2, MMR, and PD-L1 expression with patient prognosis.
Clinicopathological features Events
(n)Mean DFS
(months) ± SDχ2 p-value ERBB2 status 0.057 0.812 Negative (−) 48 40.312 ± 17.40 Positive (+) 9 38.003 ± 13.73 MMR status 0.009 0.926 pMMR 50 40.67 ± 14.36 dMMR 7 40.18 ± 14.49 PD-L1 expression 5.898 0.015* Negative (−) 32 41.08 ± 17.27 Positive (+) 25 40.31 ± 17.40 * p < 0.05 indicates statistical significance. -
ERBB2 amplification rates vary globally, 12%−13%[8,9], and the observed rate in 14.8% of the present study cohort aligns with Asian epidemiological data. The modern anti-ERBB2 agents like trastuzumab have transformed ERBB2+ gastric adenocarcinoma (GAC) into a targetable subtype. The KEYNOTE-811 trial[10] established that adding pembrolizumab to trastuzumab/chemotherapy significantly improved ORR, PFS, and OS in RBB2+ GAC. This synergy arises from ERBB2-driven immune activation: ERBB2 amplifies tumors that exhibit enriched alanine-aspartate-glutamate (AAG) metabolism, suppressing glycolysis (GG)-associated inflammation and potentiating immune evasion. With targeted therapy, the combination of targeted therapy and immunotherapy may have a better effect.
Consequently, treatment strategies for ERBB2-positive gastric cancer are increasingly shifting toward a combination approach, reflecting the advancement of precision medicine and individualized treatment strategies.
dMMR: a gateway to immunotherapy response
-
Microsatellite instability is closely associated with mutations in mismatch repair (MMR) genes, including MLH1, MSH2, MSH6, and PMS2. Previous studies have reported that dMMR gastric cancer is more frequently observed in elderly female patients and is predominantly associated with intestinal-type histology[11,12]. dMMR prevalence correlates strongly with female predominance, moderate differentiation—consistent with prior reports. dMMR fuels immunotherapy sensitivity via: High neoantigen burden: Frameshift mutations from MMR deficiency (MLH1/MSH2 loss) trigger cytotoxic T-cell recruitment. Additionally, dMMR fuels immunotherapy sensitivity: dMMR tumors are often characterized by high PD-L1 expression[13], making dMMR status a crucial predictive biomarker for the efficacy of immune checkpoint inhibitors. Given the strong correlation between dMMR status and response to immunotherapy, assessing dMMR status is essential for optimizing treatment strategies in gastric cancer patients.
ERBB2-dMMR interplay: mutual existence and metabolic heterogeneity
-
The ERBB2 gene regulates tumor proliferation, apoptosis, and other cellular processes by forming heterodimers with members of the epidermal growth factor receptor (EGFR) family[14]. dMMR, on the other hand, is often linked to tumor immune evasion mechanisms. Investigating the potential relationship between ERBB2 amplification and MMR status holds significant clinical and biological relevance. ERBB2+ tumors exhibit AAG metabolic dominance, while dMMR favors GG metabolism with high immune infiltration and inflammation.
At the molecular level, ERBB2 amplification is closely related to DNA repair mechanisms, particularly the normal function of the MMR system. Overexpression of ERBB2 in tumor cells may help maintain DNA repair capacity, thereby reducing the likelihood of dMMR development. Previous studies have suggested that high ERBB2 expression in certain cancers can enhance the activation of DNA repair mechanisms, improving cellular resistance to external damage and promoting tumor cell survival[15,16]. In rectal cancer, studies are reporting that the overexpression of ERBB2 may be negatively correlated with the defect in mismatch repair status[17]. In studies involving Chinese gastric cancer patients, no definitive association between ERBB2 expression and dMMR status has been established[18]. The findings of the present study indicate a higher proportion of microsatellite-stable (pMMR) cases among ERBB2-positive patients, suggesting a potential negative correlation between ERBB2 amplification and dMMR status. Despite mutual exclusion, dual-targeting strategies show preclinical promise:
ERBB2+ dMMR models: Trastuzumab + anti-PD-1 synergistically inhibits tumor growth by coupling HER2 blockade with immune reactivation.
ERBB2/dMMR crosstalk in PD-L1 regulation: tissue-specific immune landscapes
-
ERBB2-PD-L1 Paradox in Gastric Cancer: ERBB2-positive tumors may enhance PD-L1 expression through activation of the ERBB2 signaling pathway, thereby promoting tumor immune escape. Previous studies have demonstrated that this mechanism plays a crucial role in certain tumors undergoing immunotherapy. For instance, in breast cancer, ERBB2 activation primarily occurs via the PI3K-AKT-mTOR and RAS-RAF-MEK-ERK signaling pathways, leading to the upregulation of pro-inflammatory cytokines (e.g., IFN-γ) and ultimately inducing PD-L1 expression in both tumor and immune cells[19]. However, in gastric cancer, ERBB2-positive patients generally exhibit lower PD-L1 expression levels compared to ERBB2-negative patients[20]. The correlation between ERBB2 and PD-L1 expression appears weak, which may be influenced by multiple factors. First, differences in tumor histopathological subtypes may lead to distinct immune escape mechanisms. In some tumor types, high ERBB2 expression does not promote immune evasion through PD-L1 upregulation but instead suppresses T-cell function or induces immune tolerance. Second, the regulatory effects of ERBB2 signaling on the immune system may be modulated by the tumor microenvironment and other molecular characteristics. Additionally, the heterogeneity of immunosuppressive mechanisms and the complexity of tumor biology may contribute to the observed discrepancies. Although some studies have reported a potential correlation between high ERBB2 expression and PD-L1 levels, the findings of this study suggest the presence of unique immune escape mechanisms in specific tumor types or patient subgroups, warranting further investigation through mechanistic studies.
Conversely, dMMR-Driven PD-L1 Elevation: dMMR tumors are often associated with higher PD-L1 expression, which serves as a key immune escape mechanism. PD-L1 facilitates tumor evasion from immune surveillance by binding to the PD-1 receptor on T cells and inhibiting their activity. As a result, dMMR and high PD-L1 expression frequently coexist, conferring enhanced responsiveness to immune checkpoint inhibitors[21,22]. For instance, the CheckMate-649 clinical trial demonstrated that gastric cancer patients with a PD-L1 combined positive score (CPS) ≥ 5 who received chemotherapy in combination with nivolumab exhibited significantly improved overall survival (OS) and progression-free survival (PFS) compared to the control group[23]. The present study found that PD-L1 expression was significantly higher in the dMMR group than in the pMMR group, further validating the strong association between dMMR status and PD-L1 expression. These findings reinforce the role of dMMR as a predictive biomarker for immunotherapy efficacy.
Prognostic reclassification: from single biomarkers to dynamic models
-
The prognostic impact of ERBB2 overexpression and dMMR status remains a subject of debate. Some studies suggest that ERBB2 overexpression is generally associated with poor prognosis, particularly in breast cancer[24]. In gastric cancer, however, the advent of targeted therapies such as trastuzumab has significantly improved the survival of ERBB2-positive patients[25−27]. Similarly, dMMR status is recognized as a favorable prognostic factor in non-metastatic colorectal cancer, but its prognostic significance in advanced metastatic colorectal cancer remains complex and inconclusive[28].
In this study, univariate analysis revealed no significant correlation between ERBB2 expression, MMR status, and overall survival. However, a significant association was observed between PD-L1 expression levels and patient survival. This discrepancy may be attributed to the relatively small number of dMMR patients in the cohort, limiting the ability to fully capture the prognostic advantage conferred by immunotherapy in this subgroup. Based on these observations, it is speculated that ERBB2 and dMMR may interact in tumorigenesis, progression, and immune escape via multiple molecular mechanisms, including PD-L1 regulation, immune evasion promotion, tumor microenvironment remodeling, and DNA repair pathway activation. These interactions may collectively impact patient prognosis. While ERBB2 amplification and dMMR lacked independent prognostic predictive value, their predictive biomarker utility remains clinically significant. Integration of ERBB2 signaling, MMR function, and PD-L1 expression may enable the following: identification of synergistic therapeutic vulnerabilities, construction of precision stratification models, and the optimization of targeted-immunotherapy combinations. Future studies should further elucidate the underlying mechanisms and evaluate their potential implications for optimizing immunotherapy strategies in gastric cancer.
-
This study establishes three cardinal findings in gastric adenocarcinoma: (1) ERBB2 amplification correlates with distinct differentiation patterns, potentially influencing morphological progression; (2) dMMR status associates strongly with female sex, moderately differentiation, and elevated PD-L1 expression, defining an immunogenic phenotype; (3) PD-L1-high tumors (CPS ≥ 5) demonstrate significantly improved survival, validating its role as a predictive biomarker for immune checkpoint inhibition. The absence of direct ERBB2-dMMR interaction, possibly due to biological independence or cohort constraints. Limitations include a single-center design and a limited ICI-treated subgroup (19%), which necessitate validation in multi-center trials. Moreover, mechanistic studies should focus on the roles of ERBB2 amplification and dMMR in shaping the tumor microenvironment, modulating immune cell infiltration, and influencing therapeutic response. Therapeutic optimization: develop biomarker-stratified algorithms. These efforts will provide a more robust theoretical foundation for optimizing immunotherapy strategies and enabling adaptive therapy sequencing in precision oncology frameworks.
-
The study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board (Ethics Committee) of the First People's Hospital of Changzhou, identification number: 2023 (教) CL087-01, approval date: October 27, 2023.
We would like to express our sincere gratitude to the ethical review board of the First People's Hospital of Changzhou for their approval of this study, which laid the ethical groundwork for our research. Their guidance was invaluable. We are deeply indebted to the Changzhou Science and Technology Plan (Grant No. CE20225039) and 'The 14th Five-year plan' High-Level Health Talents Training Project of Changzhou (Grant No. 2024CZBJ001) for providing the necessary financial support. Without their funding, this research would not have been possible. We also wish to thank all colleagues who provided indirect support, offered advice during the study, or assisted in data collection in any way, though not formally part of the author list. Their contributions, though not always visible, were crucial for the success of this project. Additionally, our families deserve our heartfelt thanks for their patience and understanding throughout the research process, which allowed us to focus on our work.
-
The authors confirm their contributions to the paper as follows: study design and final draft of the manuscript writing: Zheng X, Wu J, Wu C; collection and assembly of data: Xia H, Li Z; data analysis: Zhang D, Xia H, Wang M; draft manuscript preperation: Xia H, Li Z, Zhang D, Wang M. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article, and are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xia H, Li Z, Wang M, Zhang D, Zheng X, et al. 2025. Prognostic implications of ERBB2 amplification and mismatch repair in gastric adenocarcinoma: a single-center study. Gastrointestinal Tumors 12: e015 doi: 10.48130/git-0025-0015
Prognostic implications of ERBB2 amplification and mismatch repair in gastric adenocarcinoma: a single-center study
- Received: 18 December 2024
- Revised: 31 July 2025
- Accepted: 06 August 2025
- Published online: 23 September 2025
Abstract: This study investigated the association between ERBB2 amplification and mismatch repair (MMR) status in gastric adenocarcinoma, and evaluated their impact on tumor differentiation, PD-L1 expression, and prognosis. One hundred and seventy six patients from the First People's Hospital of Changzhou (Changzhou, China) were retrospectively analyzed. ERBB2 amplification was assessed by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), with positivity defined as an ERBB2/CEP17 ratio ≥ 2.0. MMR deficiency (dMMR) was determined by loss of MLH1, MSH2, MSH6, and/or PMS2 protein expression. PD-L1 expression was quantified using the Combined Positive Score (CPS) via the 22C3 pharmDx assay. Survival analysis employed Kaplan-Meier curves with log-rank testing. Among the cohort, 26/176 patients (14.8%) were ERBB2-positive and 22/176 (12.5%) exhibited dMMR, with no significant association. ERBB2 amplification correlated with specific tumor differentiation patterns, while dMMR was associated with female sex, moderate differentiation, and elevated PD-L1 expression. Kaplan-Meier analysis demonstrated significantly improved survival in the PD-L1-positive group, indicating PD-L1 positivity as a predictor of survival benefit. Combined assessment of ERBB2 and MMR status may optimize therapeutic strategies for gastric adenocarcinoma.
-
Key words:
- ERBB2 amplification /
- Mismatch repair /
- Gastric adenocarcinoma /
- Prognosis