-
With the continuous increase in global greenhouse gas emissions, carbon dioxide (CO2) has become the primary contributor to global warming[1−3]. Therefore, developing efficient, low-energy, and scalable CO2 capture technologies is critical for achieving carbon neutrality. Among various approaches, adsorption using carbon-based materials has attracted extensive attention owing to their high surface area, chemical stability, and structural tunability[4,5]. In particular, introducing heteroatoms such as nitrogen and oxygen into carbon frameworks has been widely demonstrated to enhance CO2 affinity by increasing surface polarity, and Lewis basicity[6−9].
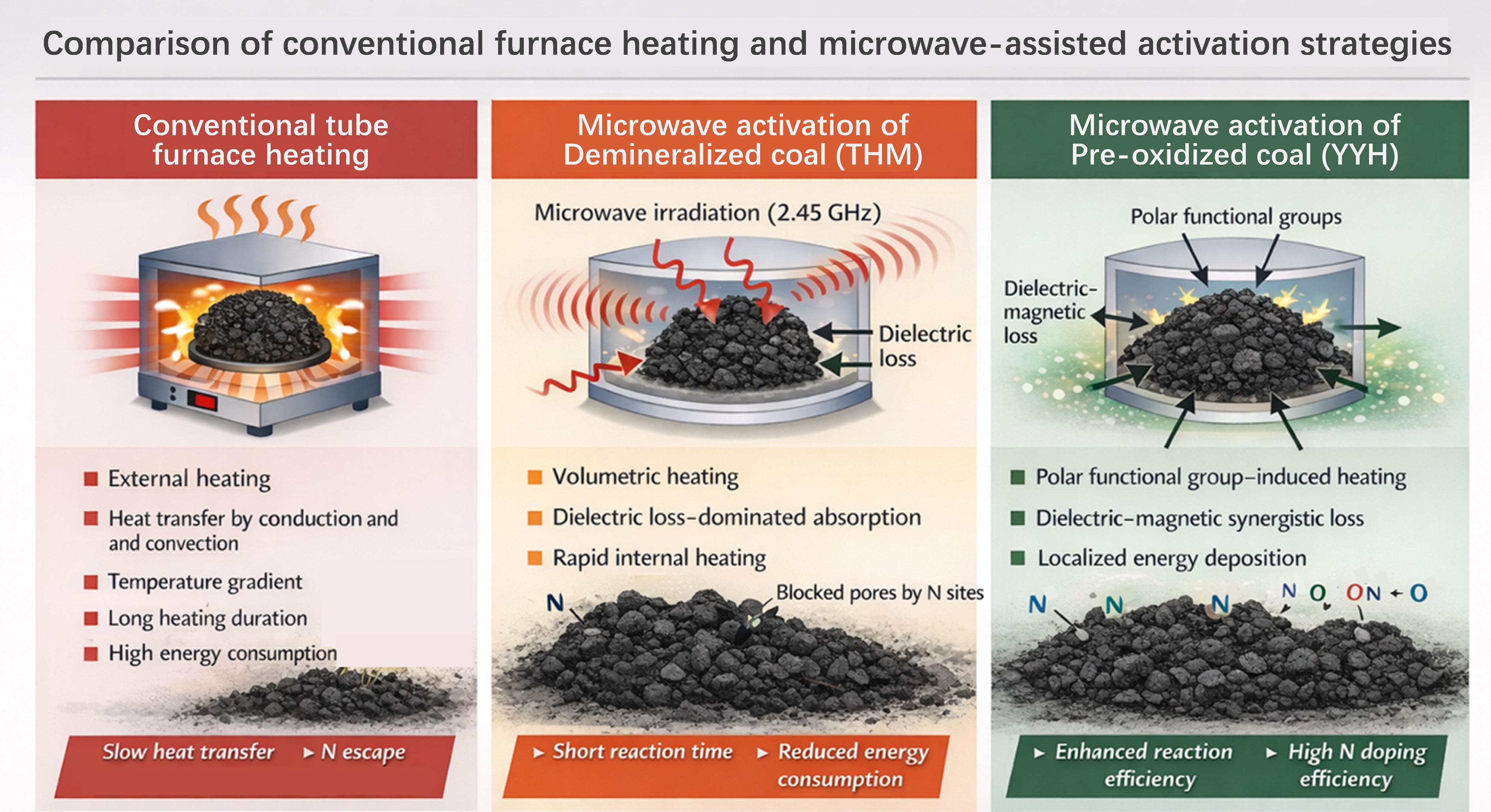
To date, most heteroatom-doped porous carbons are prepared via conventional tube-furnace heating combined with chemical activation. However, this strategy inherently relies on prolonged high-temperature treatment (> 700 °C), which inevitably causes severe volatilization of nitrogen- and oxygen-containing species below 600 °C, leading to low heteroatom retention and poor controllability of surface chemistry[10−12]. As a result, the nitrogen content of activated carbons produced by conventional heating typically remains below ~5 at.%, and further enhancement often requires excessive addition of nitrogen precursors at the expense of pore accessibility and adsorption efficiency[13].
Recent studies on heteroatom-doped biomass-derived carbons have emphasized the importance of synergistically optimizing narrow microporosity and surface functionality for CO2 adsorption. For example, N/S-co-doped carbons derived from coconut shell exhibit enhanced CO2 uptake due to the combined contribution of heteroatom-rich active sites and ultramicropores, although their synthesis still relies on conventional thermal routes with limited heteroatom utilization efficiency[14]. Similarly, boron-doped porous carbons prepared using metaborate activators demonstrate improved CO2 affinity through electronic structure modulation, yet the high activation temperatures required still restrict precise control over dopant retention and spatial distribution[15,16]. These studies highlight that, despite notable progress, the fundamental challenge of simultaneously achieving efficient heteroatom incorporation, controlled pore evolution, and energy-efficient processing remains unresolved.
Microwave-assisted heating has recently emerged as a promising alternative due to its volumetric, selective, and rapid heating characteristics[17]. Unlike conventional external heating, microwave irradiation enables direct coupling between electromagnetic energy and carbon precursors, offering the potential to accelerate pore formation while suppressing excessive heteroatom loss. Nevertheless, most existing microwave-based studies focus primarily on rapid activation or morphology evolution, while the role of precursor pre-oxidation in regulating microwave energy coupling, heteroatom substitution pathways, and pore blockage effects has rarely been systematically explored.
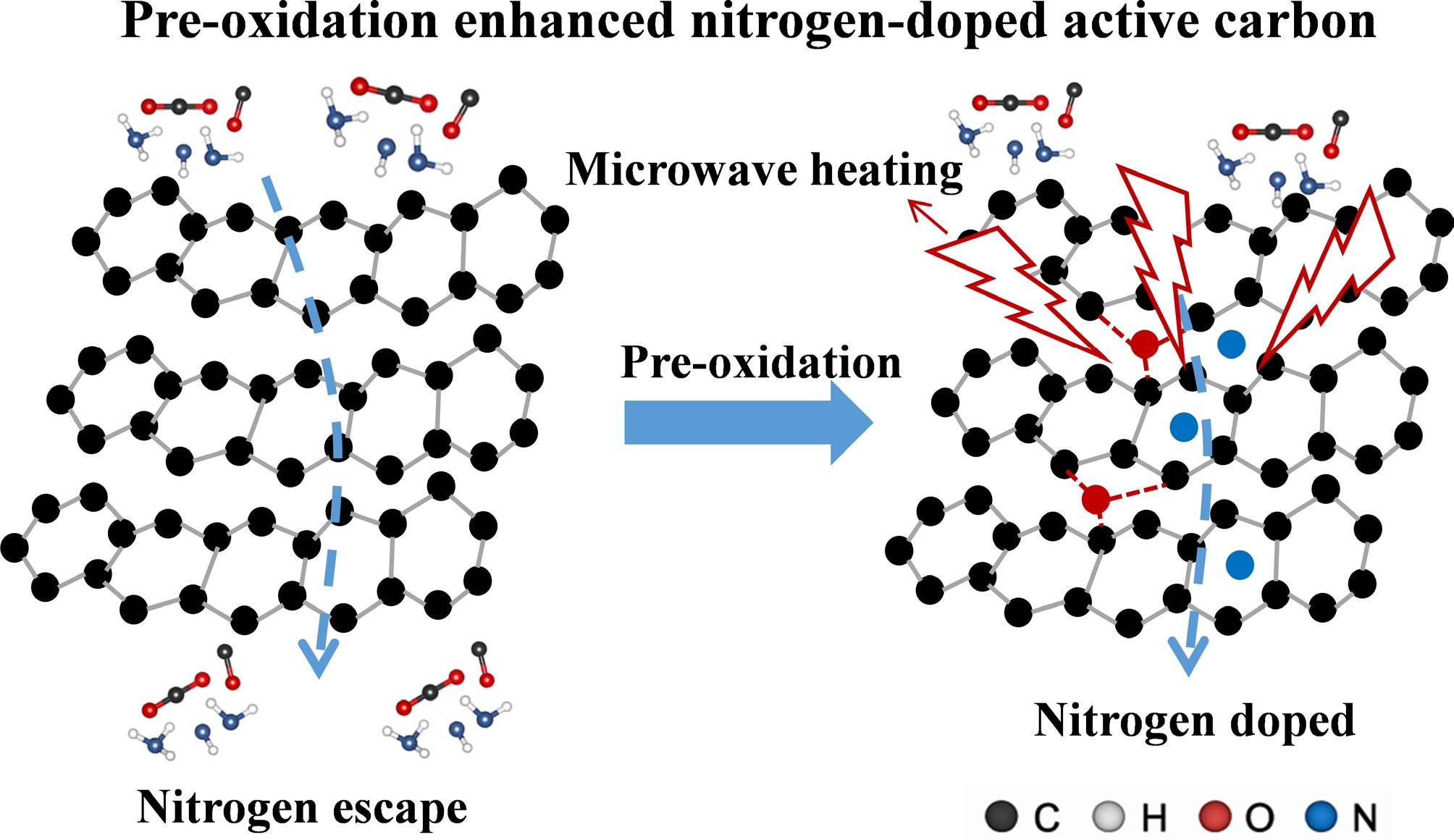
In this work, a pre-oxidation–assisted microwave activation strategy is proposed for coal-derived carbon materials. Pre-oxidation introduces abundant oxygen-containing active sites that facilitate subsequent nitrogen substitution under microwave irradiation, thereby mitigating nitrogen loss and alleviating pore blockage induced by bulky nitrogen functionalities. As schematically illustrated in Fig. 1, this strategy fundamentally differs from both conventional tube-furnace activation and direct microwave activation of raw coal, enabling more efficient energy utilization, enhanced heteroatom retention, and improved accessibility of ultramicropores. This work thus provides new mechanistic insight into the pre-oxidation–microwave synergistic regulation of heteroatom doping and pore architecture, offering a viable pathway for the rational design of high-performance CO2 adsorbents.
Herein, a scalable 'pre-oxidation + microwave activation' synergistic strategy is proposed to construct N/O co-doped ultramicroporous coal-based carbons. Benefiting from the high microwave absorption efficiency (> 90%) and volumetric heating properties, the production time of activated carbon was shortened to only 10 min. The oxygen-containing functional groups introduced by pre-oxidation facilitated oxygen-exchange reactions during microwave activation, thereby enhancing nitrogen incorporation. Interestingly, the pre-oxidized samples exhibited an opposite trend to untreated coal—wherein nitrogen content increased with temperature, reaching a maximum of 10.06 at.%. The resulting materials exhibited superior CO2 adsorption performance, achieving 4.72 mmol·g−1 at 0 °C and 1 bar, and maintaining 3.33 mmol·g−1 at 25 °C, along with excellent CO2/N2 selectivity (> 100). This work demonstrates that coupling thermochemical regulation with microwave-induced defect engineering enables simultaneous optimization of pore structure and surface functionality, offering a promising route for low-cost, high-efficiency coal-based adsorbents for carbon capture applications.
-
Ningdong coal (proximate and ultimate analyses shown in Supplementary Table S1) with a particle size range of 0.2–0.425 mm (40–80 mesh) was subjected to acid-assisted demineralization using 15 wt% HCl, and 20 wt% HF solutions to remove inherent ash and inorganic impurities, yielding a purified carbon precursor referred to as de-ashed coal (THM). The obtained THM sample was subsequently pre-oxidized in a muffle furnace at 350 °C for 3 h under an air atmosphere, producing the pre-oxidized coal precursor (YYH). Thereafter, 2 g of the carbon precursor (THM or YYH) was homogeneously mixed with 4 g of KOH, and 2 g of melamine, and the resulting mixture was placed in a quartz tube for the microwave-assisted synthesis of activated carbon. For comparative testing, a control group was also prepared under different potassium hydroxide ratios, and without melamine doping.
Experimental system
-
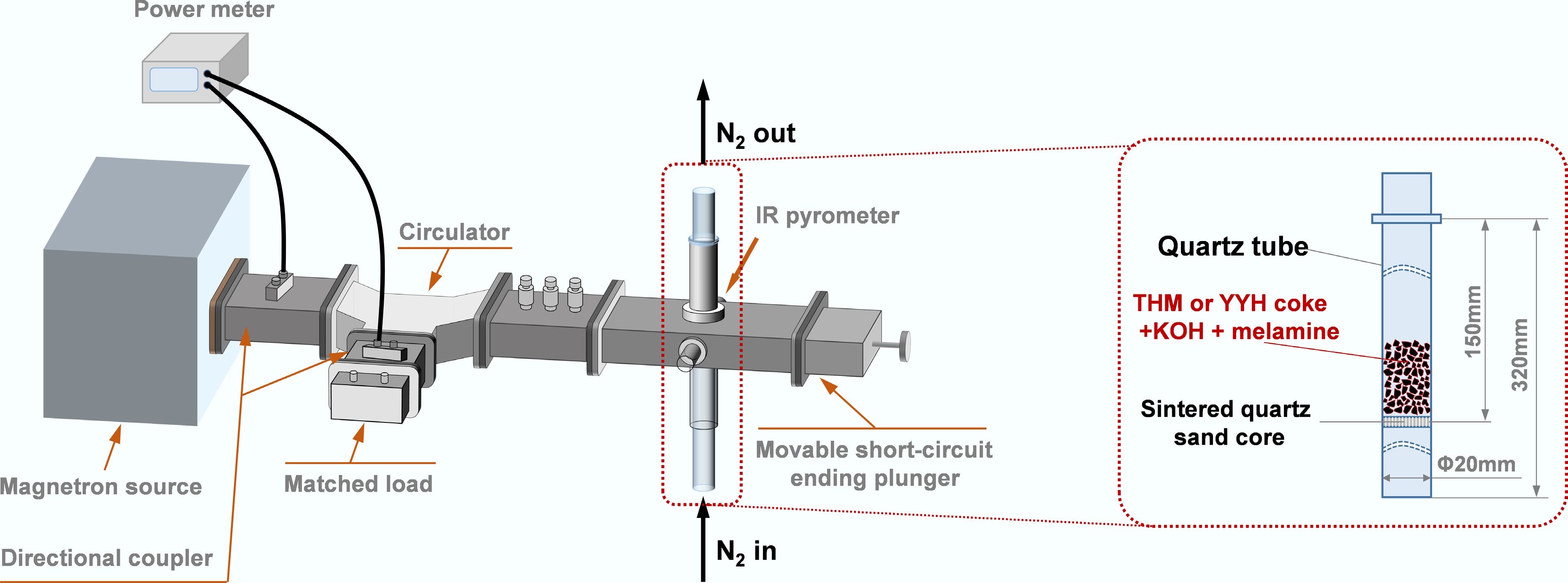
As illustrated in Fig. 2, the microwave heating apparatus consisted of a single-mode resonant cavity (ZDM-3), a fixed-bed reactor, a microwave power meter (AV 2438 CB), an infrared (IR) temperature sensor, and an N2 mass flow controller. The microwave power meter continuously monitored the incident and reflected power to calculate the absorbed microwave power and corresponding absorbed energy. Throughout the synthesis process, a constant N2 flow rate of 100 mL·min−1 was maintained, with the microwave input power set to 200, 250, or 300 W, for a fixed irradiation duration of 10 min. After synthesis, the obtained activated carbons (ACs) were thoroughly washed with deionized water to remove residual potassium-containing species. The washing process was continued until the pH of the filtrate reached neutrality, after which the samples were dried to obtain the final activated carbon products. The resulting activated carbon samples were denoted as THM-200/250/300 W, and YYH-200/250/300 W, respectively.
Structural characterization
-
The porous structures of the samples were characterized by nitrogen adsorption–desorption isotherms using a 3H2000PM2 analyzer (Beishide Instrument Technology Co., Ltd, Beijing, China). The surface morphology of the activated carbons was examined by scanning electron microscopy (SEM, SU8010). Furthermore, the physical and chemical structures of the activated carbon samples were analyzed using electron paramagnetic resonance spectroscopy (EPR, MS5000), X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI), X-ray diffraction (XRD, Rigaku SmartLab SE), Raman spectroscopy (Renishaw inVia Reflex, 532 nm excitation), and Fourier transform infrared spectroscopy (FTIR, Nicolet iZ10).
Adsorption characteristics of CO2
-
The CO2 adsorption performance of the samples was evaluated at 0 and 25 °C. For dynamic CO2 adsorption measurements, a constant CO2 feed flow rate of 2 mL·min−1 was applied at 25 °C to obtain the breakthrough curves. The dynamic adsorption characteristics were assessed based on the calculated adsorption capacity[18].
$ {q}_{\tau }=V\int\nolimits_{0}^{\tau }\left({C}_{0}-C\right)\text{d}t/{m}_{\text{bed}} $ (1) In these calculations, C0 (mg·m−3) and C (mg·m−3) represent the inlet and outlet CO2 concentrations, respectively; t (min) denotes the adsorption time; qt (g·g−1) refers to the adsorption capacity; and V represents the gas flow rate. The CO2 adsorption isotherms of 0.1 g samples were measured at 25 °C using a 3H2000 PM2 physical adsorption analyzer. The obtained isotherms were fitted using the Langmuir model (Eq. [2]), the Freundlich model (Eq. [3]), and the Sips model (Eq. [4]) to analyze the CO2 adsorption behavior of the activated carbon[19].
$ {q}_{\text{e}}={q}_{\text{m}}{k}_{\text{L}}p/\left(1+{k}_{\text{L}}p\right) $ (2) $ {q}_{\text{e}}={k}_{\text{F}}{p}^{1/{{n}_{\text{F}}}} $ (3) $ {\mathrm{q}}_{\text{e}}={q}_{\text{S}}{k}_{\text{S}}{\mathrm{C}}^{\text{1/}{{n}_{\text{S}}}}/\text{(}1+{k}_{\text{S}}{\mathrm{C}}^{\text{1/}{{n}_{\text{S}}}}\text{)} $ (4) where, p (bar), kL (bar−1), qs (mmol·g−1), kF ((bar−1)1/nF, and nF denote the equilibrium concentration, Langmuir constant, theoretical maximum adsorption capacity, Freundlich affinity constant, and the Freundlich constant, respectively. The kS ((bar−1)1/nS) and nS denote the Sips constants.
Meanwhile, N2 adsorption isotherms were also measured at 25 °C to investigate the selectivity of ACs using 0.1 g samples. The ideal adsorption solution theory (IAST) was used to calculate the adsorption selectivity of CO2/N2 (Sads)[20].
$ {S}_{\text{ads}}={q}_{1}{p}_{2}/{q}_{2}{p}_{1} $ (5) where, q1 and p1 denote the adsorption volume and the partial pressure of CO2, respectively. q2 and p2 represent the adsorption volume and the partial pressure of N2, respectively. In this work, the fitted adsorption data obtained from the adsorption isotherm model for the IAST calculations, and the concentrations of CO2 and N2 are 85% and 15 %, respectively.
The isosteric heat of adsorption of CO2 can be calculated as follows (Eq. [6]):
$ \ln (p2/p1)=-(\Delta H_{ads}/R)(1/T_2-1/T_1) $ (6) where, R denotes the universal gas constant, ΔHads denotes the isosteric heat of adsorption (kJ·mol−1), T1 and T2 are 273 and 298 K, respectively.
-
Supplementary Figure S1 shows the dielectric constants of THM and YYH coal. In a 2.5 GHz microwave reactor, both coal samples exhibited a dielectric response and underwent microwave heating. In general, raw coal exhibits weak microwave absorption at low temperatures, whereas the introduction of KOH significantly enhances its microwave absorption capability[21]. Moreover, at elevated temperatures, the microwave absorption performance of coal is further improved due to increased electronic conduction and interfacial polarization effects.
Figure 2 presents the schematic diagram of the microwave heating system, and Fig. 3a illustrates the microwave heating characteristics of different carbon precursors under various input powers. As the input power increases from 200 to 300 W, the steady-state temperature gradually rises. Under identical input power conditions, the pre-oxidized coal precursor demonstrates a shorter response time, a higher heating rate, and a higher stable temperature than the de-ashed coal precursor. As shown in Fig. 3b, c, the microwave absorption power (MAP) rapidly reaches a steady value within 4 min, while the microwave absorption efficiency (MAE) stabilizes above 90%, indicating the fast-response characteristics and remarkable energy-saving potential of the microwave heating process. Furthermore, under the same input power, the total energy consumption, represented by the absorbed microwave energy, remains comparable across samples, with a typical value of approximately 45 kJ at 250 W, as presented in Fig. 3d.
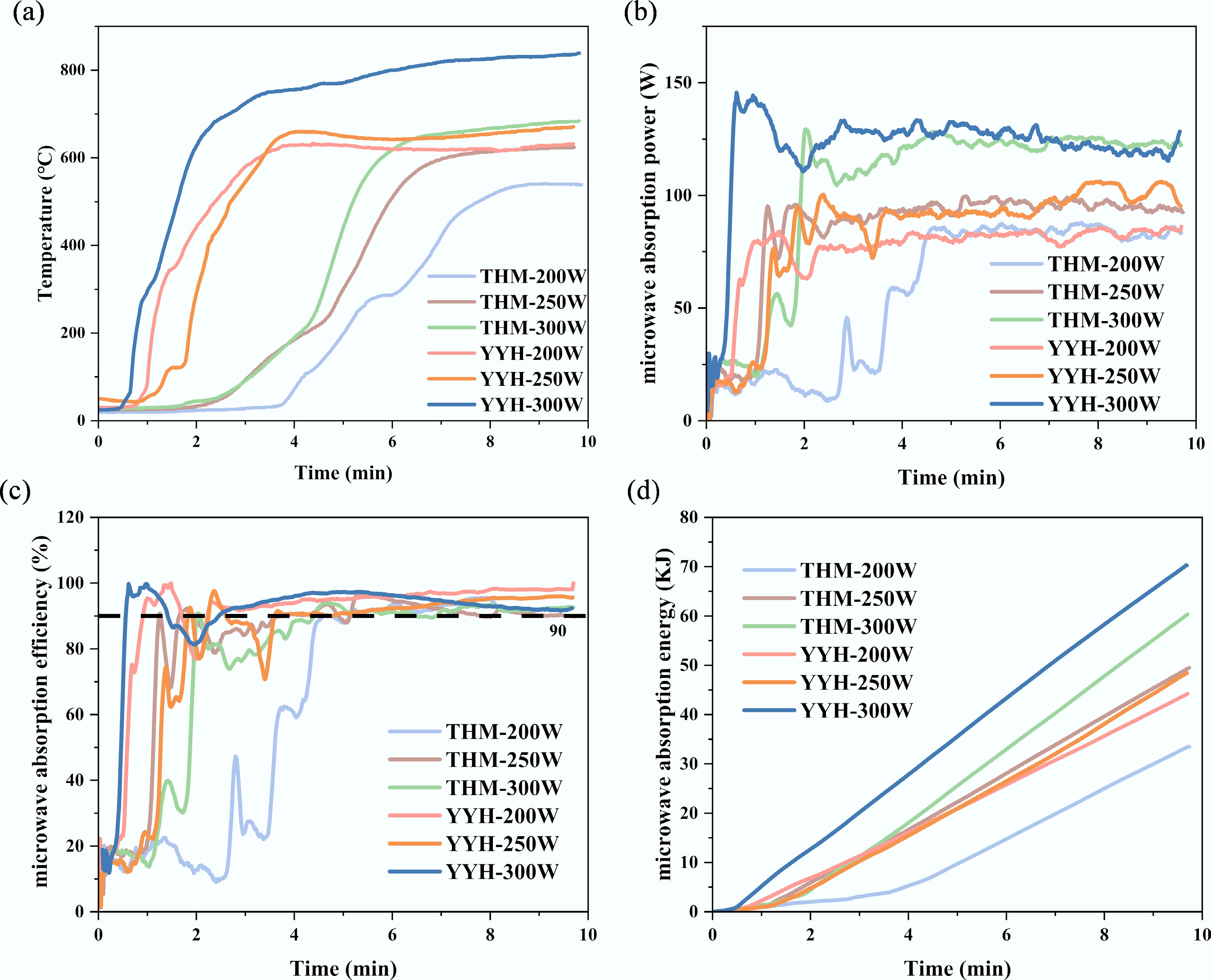
Figure 3.
Microwave heating system and heating characteristics: (a) temperature, (b) microwave absorption power, (c) microwave absorption efficiency, and (d) microwave absorption energy of different samples.
Additionally, a thermal comparison was conducted between microwave heating and conventional tube furnace heating, as shown in Supplementary Fig. S2. Compared with conventional tube-furnace heating, microwave activation exhibits significantly higher energy efficiency. Under the conditions used in this study, conventional activation was conducted in a 7 kW tube furnace for 77.5 min, resulting in a total energy consumption of approximately 9.0 kWh. In contrast, microwave activation required only 250 W for 10 min, corresponding to an energy consumption of about 0.042 kWh, which is nearly two orders of magnitude lower than that of conventional heating. This demonstrates the superiority of microwave heating.
Pore structure characterization
-
The SEM images presented in Fig. 4a reveal the formation of abundant micron-sized pores and cracks on the carbon surface. These well-developed honeycomb-like pores and fissures are attributed to the expansion and release of volatile species (e.g., NH3, H2O) generated during microwave-induced thermal decomposition within the carbon matrix[22]. For the THM series (non-pre-oxidized coal), both the pore density and pore size increase significantly with rising microwave power. The surface of THM-200W exhibits sparse and relatively closed pores, whereas THM-250W displays pronounced honeycomb-like micropores interconnected by visible cracks. At THM-300W, a more developed and uniformly distributed hierarchical pore network is observed. A similar trend is evident in the YYH series (pre-oxidized coal). However, under the same microwave power, the YYH samples show more regular pore structures and thinner, more permeable pore walls. Notably, YYH-250W exhibits improved pore size uniformity and interconnectivity compared to THM-250W, indicating that the pre-oxidation treatment promotes more orderly pore evolution during microwave activation.
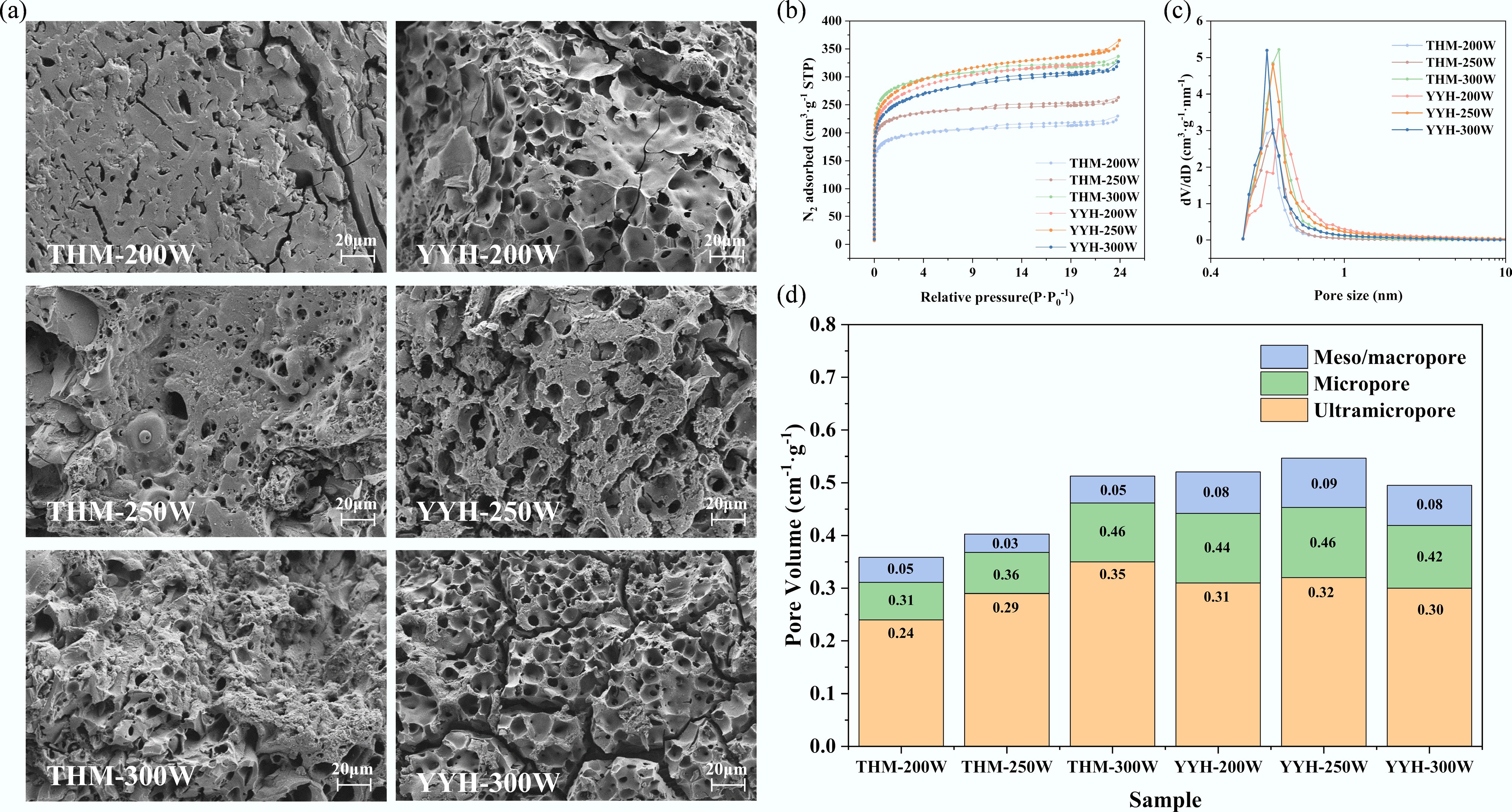
Figure 4.
The morphology and pore structure of samples: (a) SEM images, (b) N2 adsorption–desorption isotherms, (c) NLDFT pore size distributions of samples, and (d) the micropore volume, meso/macropore volume, and ultramicro volume of samples.
The six synthesized samples exhibited consistent and systematic pore structure evolution, as evidenced by both N2 adsorption–desorption measurements and SEM observations. The corresponding adsorption–desorption isotherms (Fig. 4b) display a typical mixed Type I + Type IV behavior[23]. A sharp uptake at low relative pressure (P/P0 < 0.01) indicates the presence of abundant micropores, while the appearance of a hysteresis loop in the medium-to-high pressure range signifies the existence of mesopores with diameters between 2 and 50 nm. The BET surface area increased progressively with higher microwave power and pre-oxidation treatment. Specifically, the surface area of THM-200W was 767 m2·g−1, which increased to 1,131 m2·g−1 for THM-300W. Meanwhile YYH-250W exhibited the highest value of 1,103 m2·g−1, slightly exceeding that of THM-250W (910 m2·g−1). The pore size distribution curves (Fig. 4c) show dominant peaks centered around 0.6–0.8 nm, characteristic of microporous structures. Notably, the peak positions for THM-250W and YYH-200W shift slightly toward smaller pore sizes, suggesting that the pre-oxidation treatment facilitates the generation of a greater abundance of ultramicropores within the carbon framework.
The pore volume distributions quantified by the NLDFT method (Fig. 4d) further corroborate these observations. In the THM series, the ultramicropore volume (< 0.7 nm) increased from 0.24 cm3·g−1 at 200 W to 0.35 cm3·g−1 at 300 W. For the YYH series, the ultramicropore volume reached 0.32 cm3·g−1 at 250 W, slightly higher than that of THM-250W (0.29 cm3·g−1). Meanwhile, both micropore (0.7–2 nm) and mesopore (> 2 nm) volumes exhibited a concurrent increase with rising microwave power. Although YYH-300W showed a slightly lower micropore volume (0.42 cm3·g−1) compared to THM-300W (0.46 cm3·g−1), it possessed a marginally higher mesopore volume (0.08 cm3·g−1), suggesting that pre-oxidation promotes a more balanced hierarchical pore structure conducive to gas adsorption and transport. Additionally, we performed aperture analysis on the representative sample (YYH-250W) using the DTF model, with results shown in Supplementary Fig. S3 and Supplementary Table S2. The results show a main peak at 0.6–0.7 nm, indicating that the sample is predominantly composed of ultramicroporous structures.
Accordingly, the obtained samples exhibited high microporosity. The micropore ratios of THM-200W, THM-250W, THM-300W, YYH-200W, YYH-250W, and YYH-300W were 86.62%, 91.27%, 89.94%, 84.76%, 82.99%, and 84.47%, respectively. Detailed pore structure parameters are summarized in Table 1.
Table 1. Parameters of pore structure for ACs
Sample SBET
(m2·g−1)Vmicro
(cm3·g−1)Vtotal
(cm3·g−1)Vmicro/Vtotal
(%)Vultramicro
(cm3·g−1)Vultramicro/
Vtotal (%)H–K average pore
size (nm)THM-200 767 0.31 0.36 86.62 0.24 66.61 0.70 THM-250 910 0.36 0.40 91.27 0.29 71.74 0.67 THM-300 1131 0.46 0.51 89.94 0.35 68.01 0.70 YYH-200 1057 0.44 0.52 84.76 0.31 59.27 0.70 YYH-250 1103 0.46 0.55 82.99 0.32 58.73 0.70 YYH-300 1008 0.42 0.49 84.47 0.30 60.31 0.69 As shown in Fig. 4 and Table 1, pre-oxidation (YYH series) further optimized the pore structure under identical microwave powers, resulting in enhanced ultramicroporosity and improved overall pore connectivity. Such structural refinement facilitates gas diffusion and mass transfer during subsequent dynamic adsorption processes. The synergistic effect of microwave power and pre-oxidation markedly promotes the development of a well-defined hierarchical pore architecture in the coal-based activated carbons, thereby providing an efficient pore framework for high-performance CO2 capture.
Simultaneously, throughout the entire activation process, factors such as varying KOH ratios and the inclusion or exclusion of melamine addition significantly influenced the results, in addition to microwave power. Therefore, we supplemented the above comparative experiments with results presented in Supplementary Figs S4, S5, and Supplementary Table S3.
Consequently, we ultimately concluded that the optimized conditions were coal to KOH ratio of 1:2 and the addition of melamine.The yields of activated carbon prepared at different power levels are shown in Supplementary Table S4, with values ranging from 41% to 55%. Moreover, as the microwave power increases, the yield decreases, and the yield of pre-oxidized coal is higher than that of delignified coal. This high yield demonstrates the potential for microwave-assisted carbon production in industrial applications.
Defects and surface functional groups characterization
-
Furthermore, the physicochemical structural characteristics of the synthesized activated carbons were systematically investigated to elucidate their structural evolution. Raman spectroscopy, a powerful technique for probing the degree of ordering and defect distribution in carbon materials, was employed to reveal the structural variations of sp2-hybridized carbon frameworks. As shown in Fig. 5a, all samples exhibit the characteristic dual-band Raman features: the D band (~1,350 cm−1), associated with disordered carbon (such as edge defects, lattice imperfections, or sp3-hybridized structures), and the G band (~1,580 cm−1), corresponding to the in-plane vibrations of graphitic sp2 carbon atoms[24]. With the increase of microwave power from 200 to 300 W, the intensity of the D band becomes significantly stronger while that of the G band weakens, indicating that the higher energy density of microwave irradiation promotes more intense thermal decomposition reactions, leading to the generation of additional structural defects and a decrease in the overall graphitic order. This trend demonstrates that microwave irradiation not only accelerates the carbonization process but also plays a crucial role in tailoring the microstructural organization of the carbon framework.
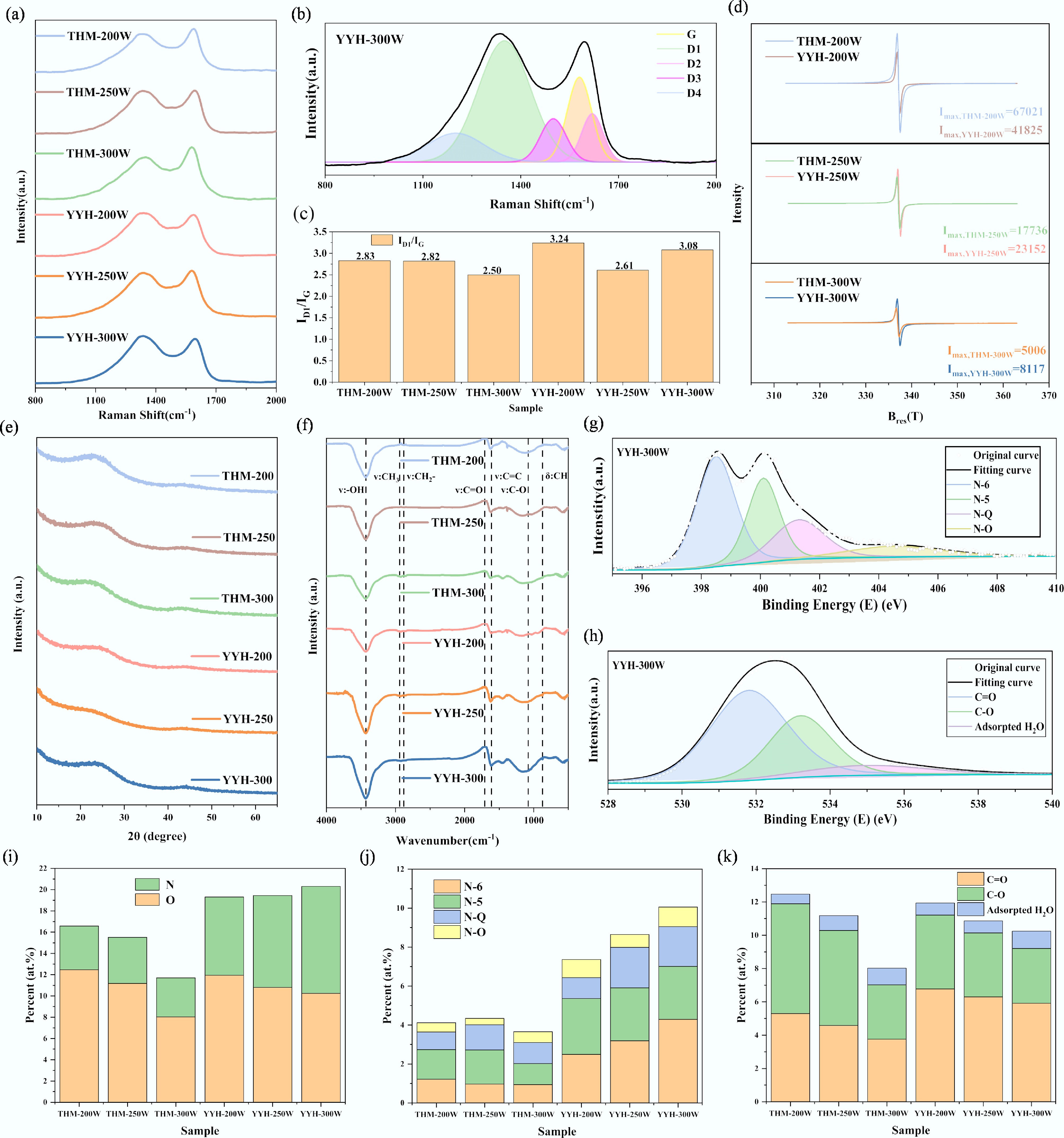
Figure 5.
Physicochemical structure characterizations: (a) Raman spectrum, (b) Raman spectrum fitted into five bands of CH-0, (c) ID1/IG, (d) EPR spectroscopy, (e) XRD spectrum, (f) FTIR spectrum, (g) deconvoluted N1s spectrum of YYH-300W, (h) deconvoluted O1s spectrum of YYH-300W, (i) nitrogen and oxygen content of samples, (j) nitrogen functional groups distributions, and (k) oxygen functional groups distributions.
As shown in Fig. 5b and Supplementary Fig. S6, the fitted spectra of the THM and YYH samples further elucidate the complex structural composition within the carbon materials. Specifically, the five sub-bands correspond to graphitic ordered carbon (G band), edge-defect carbon (D1), surface impurity-related carbon (D2), amorphous carbon (D3), and oxygen-containing functionalized carbon structures (D4)[25]. With increasing microwave power, the THM series exhibits a pronounced enhancement in the D1 and D3 bands, accompanied by a gradual attenuation of the G band, indicating that high-energy microwave irradiation induces significant structural disordering.
In contrast, the YYH series (pre-oxidized samples) displays stronger D1, D3, and D4 features under identical power levels, most notably in YYH-300W, suggesting that pre-oxidation promotes structural activation, amorphous carbon enrichment, and the formation of edge-related sites. Although increased disorder disrupts the graphitic ordering to some extent, it simultaneously introduces a higher density of active sites and surface defects, which can exert a favorable influence on CO2 adsorption[26]. Moderate structural disorder enhances gas affinity and diffusion kinetics, thereby improving both adsorption capacity and dynamic performance. However, excessive defect generation or over-disordering may lead to pore collapse and surface area reduction, ultimately hindering adsorption efficiency. Therefore, the controlled regulation of microwave power and pre-oxidation conditions to achieve a state of moderate disorder in the carbon structure is crucial for optimizing CO2 adsorption performance.
For the Raman spectra, the intensity ratio of the D1 band to the G band (ID1/IG) is commonly employed to evaluate the degree of disorder or defect density in activated carbons, as shown in Fig. 5c[27]. Compared with the non-pre-oxidized THM series, the YYH series (pre-oxidized) exhibits higher ID1/IG ratios under identical microwave power conditions, indicating that pre-oxidation further disrupts the graphitic framework and enhances the structural disorder of the carbon materials. Notably, molecular dynamics studies have demonstrated that an increased defect concentration facilitates heteroatom doping, which in turn contributes to the stabilization of the carbon framework[28]. Therefore, the high defect density induced by microwave irradiation provides a favorable environment for subsequent heteroatoms incorporation.
Figure 5d presents the EPR responses of the samples under different microwave powers. The results indicate that, with increasing microwave power, the peak intensity of the EPR signals for both the THM and YYH series gradually decreases, suggesting that some electron defects undergo reconstruction or 'healing' under high-energy microwave irradiation, leading to a more stabilized carbon framework and reduced defect activity[29]. Moreover, at 250 and 300 W, the Ipeak,EPR of the YYH samples is notably higher than that of the corresponding THM samples, implying that pre-oxidation introduces additional initial radical sites, which are more responsive under microwave excitation. This effect is particularly pronounced at medium-to-high power levels, indicating that the pre-oxidation treatment promotes defect formation in the carbon matrix.
XRD is widely used in the carbon materials field to evaluate both the stacking order of carbon layers and the variations in graphite microcrystal size, interlayer spacing, and overall carbon framework ordering[30]. In this study, as shown in Fig. 5e, all samples exhibit two characteristic diffraction peaks at approximately 23° and 43°, corresponding to the (002) and (100) planes of the carbon materials, respectively. The (002) plane reflects the stacking structure of graphite layers and serves as an important indicator of graphitization degree, whereas the (100) plane represents the in-plane atomic arrangement of graphite crystallites[31]. The broad and weak nature of the (002) diffraction peaks across all samples indicates that the materials are predominantly amorphous carbon with only minor, low-degree graphitized microcrystalline structures. This observation suggests that under the employed microwave treatment conditions, although carbonization and structural reorganization occur, highly ordered graphite structures are not formed, and the carbon framework remains largely disordered. These findings are consistent with the Raman and EPR analyses, collectively revealing the structural evolution of the carbon materials under different processing conditions.
The microcrystalline structural parameters, including La, Lc, and d002, were further calculated using the Bragg and Scherrer equations to quantify the lateral size, vertical stacking height, and interlayer spacing of the carbon layers, thereby assessing changes in structural ordering of the carbon materials[32]. As summarized in Table 2, within the THM series, increasing the microwave power from 200 to 300 W led to a decrease in d002 from 0.38 to 0.35 nm, followed by a slight rebound, while La decreased from 4.43 to 3.40 nm. This indicates that moderate microwave power promotes denser stacking of carbon layers and enhances structural ordering, whereas excessively high power may cause partial ablation or disturbance of the layers, resulting in a slight lateral expansion. The Lc values remained relatively constant (0.94–1.00 nm), reflecting stable layer stacking numbers. In contrast, the pre-oxidized YYH series exhibited overall larger d002 values (up to 0.39 nm for YYH-250W), and smaller La values (minimum of 3.06 nm for YYH-250W), indicating that pre-oxidation introduces oxygen-containing functional groups that disrupt π–π stacking between carbon layers, weaken the regularity of microcrystalline structures, and further promote structural disorder.
Table 2. Physicochemical structural parameters of ACs
Sample ID1/IG La (nm) Lc (nm) d002 (nm) Ipeak,EPR THM-200 2.83 4.43 0.94 0.38 67,021 THM-250 2.82 3.40 1.00 0.35 17,736 THM-300 2.50 3.49 0.95 0.38 5,006 YYH-200 3.24 4.15 1.03 0.37 41,825 YYH-250 2.61 3.06 0.92 0.39 23,152 YYH-300 3.08 3.51 0.97 0.38 8,117 These XRD trends are consistent with the Raman spectroscopy results, specifically, the increase in the ID1/IG ratio, and the enhanced defect concentration observed in EPR analyses. Together, these three characterization techniques reveal a coherent structural evolution pathway. Under the synergistic influence of microwave irradiation and pre-oxidation, the carbon framework gradually transforms from partially ordered graphite microcrystals to disordered amorphous carbon. Notably, in YYH-250W, the expansion of d002, reduction of La, decrease in ID1/IG, and a simultaneous peak in free radical concentration indicates that the microcrystalline carbon has been deconstructed to a critical activation state.
Furthermore, Fourier-transform infrared (FTIR) spectroscopy was employed to analyze the surface chemical structures of activated carbon samples prepared under different microwave power densities, to elucidate the effect of microwave regulation on the evolution of surface functional groups, as shown in Fig. 5f. The FTIR spectra can be divided into four characteristic absorption regions: 3,600–3,000 cm−1, corresponding to hydroxyl (–OH) stretching vibrations, 3,000–2,700 cm−1, associated mainly with aliphatic hydrocarbons (–CH3, –CH2) vibrations, 1,800–1,000 cm−1, representing various oxygen-containing functional groups (e.g., carbonyl C=O, ester C–O–C, and carboxyl groups), and 900–700 cm−1, attributed to aromatic ring skeletal vibrations indicative of aromatic carbon structures[33].
Distinct differences in the surface functional composition among the six samples reveal that both microwave power and pre-oxidation significantly influence the reconstruction of surface chemistry. All samples display pronounced –CH3/–CH2 (aliphatic), and =CH (aromatic) vibration peaks around 2,900 cm−1 and below 3,000 cm−1, confirming the coexistence of aliphatic hydrocarbons and aromatic carbon layers within the coal-based carbon framework[31]. In the THM series, strong carbonyl (C=O, ~1,700 cm−1), and carboxyl-related peaks are observed at low power (200 W), indicating that the unoxidized coal retains abundant oxygen-containing groups such as carboxylic acids and ketones under mild treatment. As the power increases to 250 and 300 W, the intensity of the C=O-related peaks rapidly diminishes or even vanishes, suggesting that higher power induces more severe pyrolysis, resulting in the removal, cleavage, or condensation of oxygenated groups, and promoting deoxygenation and aromatization of the carbon framework.
In contrast, the YYH series (pre-oxidized samples) exhibits no distinct C=O absorption peak in its FTIR spectra but displays a strong hydroxyl (O–H) vibration near 3,400 cm−1. Combined with the enhanced C–O absorption in the 1,200–1,000 cm−1 region, this indicates that pre-oxidation promotes the transformation of carboxyl and carbonyl groups into phenolic and ether-like structures, thereby improving the hydrophilicity and thermal stability of the carbon materials. This transformation may also stabilize the hydroxyl signal through hydrogen bond formation.
To further elucidate the influence of different microwave powers and pre-oxidation treatments on the surface chemical properties of coal-based activated carbons, X-ray photoelectron spectroscopy (XPS) was employed to systematically analyze the elemental composition, and functional group structures of the prepared samples[34]. The survey spectra (Supplementary Fig. S7) indicate that all samples primarily contain C, O, and N elements, exhibiting clear heteroatom-doping characteristics.
The core purpose of peak fitting is to resolve shifts in binding energy within the core levels (e.g., C 1s, O 1s, and N 1s) corresponding to different chemical environments, thus enabling precise identification of characteristic functional groups such as C=O, C–O, C–N, pyridinic N (N-6), and pyrrolic N (N-5)[18]. The results reveal that both microwave power and pre-oxidation treatment significantly affect the bonding configurations and relative contents of heteroatoms (N and O), thereby modulating the surface electronic environment and distribution of active sites in the carbon framework. These variations directly influence the strength of interactions between activated carbon and gas molecules such as CO2, ultimately determining its adsorption performance and selectivity. Accordingly, the XPS peak-fitting spectra (Supplementary Fig. S8) not only provide direct evidence for the doping mechanisms of heteroatoms within the carbon lattice but also offer essential theoretical insight into the structure–property relationship between surface functional group regulation and adsorption behavior. Thus, XPS serves as a crucial analytical tool for probing the microstructural evolution of functionalized porous carbon materials[35].
The THM and YYH series samples exhibited pronounced differences in the elemental composition of C, O, and N, reflecting the distinct structural effects of microwave power and pre-oxidation treatment. In the THM series, as the microwave power increased from 200 to 300 W, the nitrogen content slightly decreased from 4.12 at.% to 3.67 at.%, while the oxygen content declined more significantly from 12.47 at.% to 8.03 at.%. This trend indicates that high-power microwave irradiation induces thermal decomposition or desorption of N- and O-containing functional groups, consistent with the disappearance of the C=O peak and the attenuation of C–O absorption bands observed in FTIR spectra.
In contrast, the YYH series samples, subjected to pre-oxidation, demonstrated a notable enhancement in heteroatom incorporation. The nitrogen content increased from 7.36 at.% to 10.06 at.%, markedly higher than that of the THM counterparts, while the oxygen content remained above 10 at.% (as demonstrated in Fig. 5g & h). These results suggest that the synergistic effect of pre-oxidation and microwave treatment effectively promotes co-doping of nitrogen and oxygen, thereby enhancing the surface polarity and chemical reactivity of the carbon materials. The detailed quantitative data on nitrogen- and oxygen-containing functional groups for all activated carbon samples are summarized in Table 3.
Table 3. Nitrogen and oxygen functional group contents of ACs
Sample C1s (at.%) O1s (at.%) N1s (at.%) C=O (at.%) C–O (at.%) N-6 (at.%) N-5 (at.%) N-Q (at.%) N-O (at.%) THM-200 83.41 12.47 4.12 5.3 6.59 1.22 1.52 0.9 0.48 THM-250 84.47 11.18 4.34 4.58 5.71 0.97 1.75 1.29 0.33 THM-300 88.3 8.03 3.67 3.76 3.26 0.94 1.08 1.08 0.56 YYH-200 80.69 11.95 7.36 6.78 4.43 2.49 2.87 1.07 0.93 YYH-250 80.55 10.8 8.65 6.3 3.84 3.19 2.72 2.08 0.66 YYH-300 80.81 10.25 10.06 5.92 3.29 4.29 2.72 2.04 1.01 As shown in Table 3, and the functional group distribution diagrams of activated carbons (Fig. 5i, k), distinct trends are observed in the surface chemical compositions. In the THM series, the dominant oxygen-containing groups are C=O and C–O species, with the proportion of C=O gradually decreasing as microwave power increases, confirming the thermal instability of carboxyl and carbonyl functionalities under high-energy conditions[24]. In contrast, the YYH series exhibits a generally higher C=O content, suggesting that the pre-oxidation treatment promotes the enrichment of oxidized fragments. Meanwhile, the lower proportion of C–O bonds implies that certain hydroxyl groups may have been converted into carbonyl structures or further aromatized.
Regarding nitrogen functionalities, four main types are identified: pyridinic-N (N-6), pyrrolic-N (N-5), graphitic-N (N-Q), and oxidized-N (N-O). In the THM series, N-6 and N-5 species are relatively scarce and dispersed. Conversely, the YYH series maintains a stable combined proportion of N-6 and N-5 above 5%, with particularly high concentrations in YYH-250W and YYH-300W (N-6 = 4.29%, N-5 = 2.72%). This indicates that pre-oxidation facilitates heteroatom incorporation at edge and vacancy sites, forming stable p-type dopant configurations. Such findings are consistent with the enhanced EPR defect signals, confirming that the synergistic effect of microwave irradiation and pre-oxidation provides preferential pathways for nitrogen doping at defect-rich locations.
In summary, the synergistic effect of pre-oxidation and microwave treatment effectively promotes nitrogen–oxygen co-doping, thereby enhancing the surface polarity and density of chemically active sites in the activated carbons. The nitrogen species are predominantly composed of pyridinic-N and pyrrolic-N, both of which exhibit strong electron-donating capability and high CO2 affinity, providing a favorable structural foundation for subsequent adsorption enhancement. Compared with the untreated THM series, the YYH series not only maintains a higher nitrogen content but also demonstrates a more rational distribution of surface functional groups. This indicates that the combined pre-oxidation and microwave approach is an effective strategy for constructing highly polar and defect-enriched carbon frameworks, serving as a key route toward nitrogen–oxygen functionalization.
To further investigate the differences between the THM and YYH experimental groups, changes in surface oxygen-containing functional groups during the pre-oxidation process were examined. XPS and FTIR analyses were conducted on the raw coal samples THM and YYH. Supplementary Table S5 lists the elemental compositions of the two samples determined by XPS analysis. Supplementary Fig. S9 displays the peak patterns of oxygen functional groups in the XPS spectra, while Supplementary Fig. S10 presents the FTIR spectra. The results consistently reveal that pre-oxidation markedly increases the abundance and stability of oxygen-containing functional groups in YYH. Compared with THM, YYH exhibits higher O1s content and a significantly increased proportion of thermally stable C=O groups, accompanied by the depletion of aliphatic C–H structures and reconstruction of the aromatic framework. These results demonstrate that pre-oxidation transforms unstable moieties into polar carbonyl functionalities, providing a favorable structural basis for efficient microwave energy coupling and subsequent reaction activation. Consequently, this leads to the pronounced differences in physicochemical properties observed in the aforementioned samples.
CO2 adsorption characteristics
-
Furthermore, CO2 adsorption experiments were conducted to evaluate the adsorption performance of the synthesized activated carbons. Figure 6a presents the CO2 adsorption isotherms at low temperature, while Fig. 6b shows those measured at ambient temperature. Evidently, the CO2 adsorption capacity of the activated carbons increases with the enhancement of pore volume (Vmicro and Vtotal), and nitrogen content. The CO2 uptake at low temperature follows the order: YYH-250W > YYH-300W > YYH-200W > THM-300W > THM-250W > THM-200W. Whereas at ambient temperature, the order is YYH-250W > THM-300W > YYH-300W > YYH-200W > THM-250W > THM-200W. These trends indicate that ultramicropore volume primarily governs CO2 adsorption capacity, while pore size distribution and the contents of nitrogen and oxygen functional groups also exert significant influence. Notably, YYH-300W possesses a Vmicro that is 91.3% of that of THM-300W, yet exhibits a higher CO2 adsorption capacity, further confirming the crucial role of N/O co-doping in CO2 capture. Moreover, although YYH-300W exhibits smaller SBET, Vmicro, and Vtotal values compared with YYH-200W, its CO2 uptake is superior, which can be attributed to its higher nitrogen content (10.06 at.%), suggesting that nitrogen functionalities significantly enhance CO2 affinity.
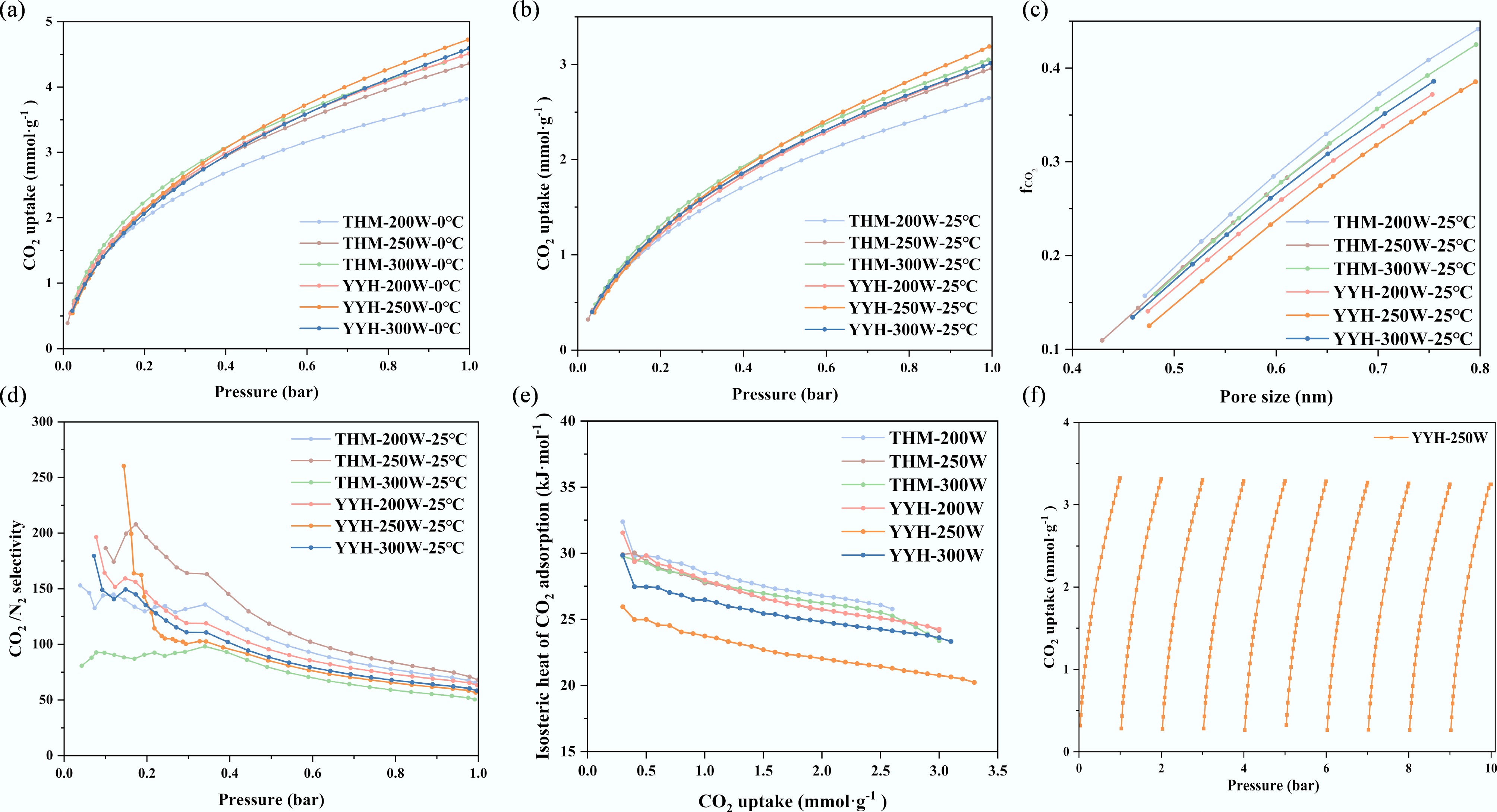
Figure 6.
The adsorption characteristics of CO2. CO2 adsorption isotherms at (a) 0 °C, and (b) 25 °C, (c) the fraction of micropore filling by CO2 ($f_{\rm CO_2} $), (d) the CO2/N2 selectivity at 25 °C and 0–1 bar (the CO2/N2 concentration ratio is 15:85); (e) The isosteric heat of CO2 adsorption; (f) cycle adsorption curve of the sample YYH-250.
Meanwhile, the influence of pore size distribution can be further elucidated by calculating the fraction of ultramicropores filled with CO2. As shown in Fig. 6c and Table 4, the
$f_{\rm CO_2} $ Table 4. CO2 adsorption characteristics of ACs
Sample CO2/0 °C CO2/25 °C N2/25 °C ${\boldsymbol f}_{\bf{ CO}_{\bf 2}} $ CO2/N2 selectivity (1 bar) THM-200 3.82 2.65 0.23 0.37 65 THM-250 4.36 2.96 0.25 0.36 68 THM-300 4.37 3.05 0.34 0.36 50 YYH-200 4.51 3.01 0.27 0.34 63 YYH-250 4.72 3.33 0.32 0.32 57 YYH-300 4.59 3.01 0.29 0.35 58 In comparison across different samples, THM-200W exhibits the highest
$f_{\rm CO_2} $ $f_{\rm CO_2} $ $f_{\rm CO_2} $ According to the ideal adsorbed solution theory (IAST), the calculated CO2/N2 selectivity (Sads) decreases with increasing pressure, as illustrated in Fig. 6d[18]. All six samples exhibit Sads values greater than 50, further confirming the excellent CO2/N2 adsorption selectivity of N/O co-doped activated carbons. These results indicate that the simultaneous optimization of pore structure and surface chemistry is an effective strategy for designing high-performance CO2 adsorbents.
Figure 6e reveals the Qst-q curves of the samples. The results indicate that the Qst value gradually decreases with increasing adsorption load, revealing the energy heterogeneity of the adsorption sites. The relatively high initial Qst value demonstrates the adsorbent's strong affinity for CO2 at low coverage. Concurrently, the representative sample YYH-250W exhibits the lowest adsorption heat. Meanwhile, Fig. 6f demonstrates the sample's excellent cycling performance, retaining 97.6% adsorption efficiency after ten cycles.
-
This work proposes a coupled strategy based on pre-oxidation pretreatment and microwave activation to efficiently construct nitrogen-doped ultramicroporous coal-based activated carbons, offering a new approach for multiscale structural synergy and optimization of gas adsorption performance. The detailed results are as follows:
(1) Benefiting from the high microwave absorption efficiency and volumetric heating characteristics, the activation time was significantly reduced to within 10 min. With increasing microwave power, both the specific surface area (up to 1,131 m2·g−1), and the proportion of ultramicropores increased markedly. Among all samples, YYH-250W exhibited the most optimized pore structure (Vtotal = 0.55 cm3·g−1).
(2) Microwave activation increased the defect density and surface active sites of the carbon framework, while pre-oxidation promoted synergistic N/O co-doping. The nitrogen content in the YYH series increased from 7.36 at.% to 10.06 at.%, with a targeted enhancement in pyridinic-N and pyrrolic-N proportions, forming electron-rich adsorption centers. Oxygen species mainly existed in C=O and C–O configurations, improving surface polarity.
(3) The YYH-250W sample exhibited the best CO2 adsorption performance, achieving a CO2 uptake of 4.72 mmol·g−1 at 0 °C and 1 bar, and maintaining 3.33 mmol·g−1 at 25 °C, with a CO2/N2 selectivity exceeding 50. The superior performance can be attributed to the synergistic effect of 0.6–0.7 nm ultramicropores and high N/O content, which collectively enhance CO2 affinity and adsorption capacity.
Overall, the results demonstrate that coupling microwave activation with pre-oxidation pretreatment enables simultaneous optimization of pore architecture and surface chemistry, yielding coal-based activated carbons with high selectivity and outstanding CO2 capture performance.
-
The authors confirm their contributions to the paper as follows: Yulin Feng: conceptualization, data curation, validation, writing − original draft; Xiaoxiao Meng: supervision, conceptualization, writing − review & editing; Jingyu Li: conceptualization, visualization, data curation; Naiyuan Xue: investigation; Wanjing Li: software; Miaoting Sun: software; Jiaxiang Chen: visualization; Xingxing Wang: data curation; Ruida Zhou: investigation; Wenjun Zhuang: methodology; Jihui Gao: project administration; Guangbo Zhao: funding acquisition; Wei Zhou: project administration. All authors reviewed the results and approved the final version of the manuscript.
-
It accompanies this paper at: https://doi.org/10.48130/scm-0026-0001.
-
The datasets used or analyzed during the current study are available from the corresponding authors upon reasonable requests.
-
This work was financially supported by the National Natural Science Foundation of China (Grant No. U21A2062).
-
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
-
Coal-based activated carbon is rapidly synthesized by microwave heating within 10 min.
A high fraction of ultramicropores (0.6–0.7 nm) is constructed for efficient CO2 confinement.
Pre-oxidation–induced oxygen substitution enables ultrahigh nitrogen doping up to 10.06 at.%.
The optimized carbon exhibits a CO2 uptake of 3.33 mmol·g−1 at 25 °C with high CO2/N2 selectivity.
-
Full list of author information is available at the end of the article.
- The supplementary files can be downloaded from here.
- Copyright: © 2026 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Feng Y, Meng X, Li J, Xue N, Li W, et al. 2026. Rapid microwave synthesis of nitrogen-doped ultramicroporous coal-based carbon with enhanced CO2 adsorption performance. Sustainable Carbon Materials 2: e006 doi: 10.48130/scm-0026-0001
Rapid microwave synthesis of nitrogen-doped ultramicroporous coal-based carbon with enhanced CO2 adsorption performance
- Received: 13 November 2025
- Revised: 05 January 2026
- Accepted: 07 January 2026
- Published online: 04 February 2026
Abstract: Ningdong coal was used as the raw material to develop a synergistic 'pre-oxidation + microwave activation' strategy for the preparation of nitrogen/oxygen co-doped ultramicroporous coal-based activated carbons (ACs), for efficient CO2 adsorption. By tuning the microwave power and pretreatment conditions, the microcrystalline structure, pore size distribution, and surface functional groups of the ACs were precisely controlled. The results show that pre-oxidation significantly enhances nitrogen retention and doping efficiency, forming CO2-philic active sites dominated by pyridinic and pyrrolic nitrogen, with a maximum nitrogen content of 10.06 at.%. Meanwhile, a large number of ultramicropores (0.6–0.7 nm) were constructed, which effectively improved the CO2 adsorption capacity and selectivity. The optimized sample achieved CO2 uptake of 4.72 mmol·g−1 at 0 °C and 1 bar, and maintained 3.33 mmol·g−1 at 25 °C, with CO2/N2 selectivity greater than 50. This work provides theoretical guidance and a practical pathway for the structure–function co-regulation and carbon capture applications of low-cost coal-derived ACs.