-
Perchlorate (ClO4−) is an inorganic ion that persists in the environment with origins of both natural and anthropogenic sources, and the latter prevalently originates from unintended leakage during extensive applications in industries including military defense, aerospace, manufacturing, and firework production[1−3]. It can easily contaminate surface water and groundwater, due to its high-water solubility, mobility, and stability[4,5]. For instance, the extensive production of fireworks, particularly in regions such as the Xiangjiang River Basin (China), generates considerable industrial wastewater characterized by extremely high concentrations of perchlorate (usually exceeding 1,000 mg·L−1), and poses a considerable threat to downstream aquatic ecosystems and drinking water sources[6,7]. Although perchlorate is readily excreted by organisms, it is considered to be a health hazard due to its adverse effects on mammalian thyroid gland function, which blocks iodide uptake and reduces the secretion of thyroid hormones[8−10]. In response to these concerns, the WHO Guidelines for Drinking-Water Quality and China's Latest National Standard for Drinking Water GB 5749-2022 explicitly established a maximum allowable concentration of 70 μg·L−1 for perchlorate in drinking water. Thus, effective and frequent detection is critical for mitigating the environmental and health impacts of this pollutant.
Various analytical techniques have been employed for perchlorate determination, including gravimetry, atomic absorption spectrometry (AAS), ion chromatography (IC), liquid chromatography-tandem mass spectrometry (LC-MS/MS), and electrochemical sensing methods such as ion-selective electrodes (ISEs)[11−15]. Among these, ISEs stand out for their promising alternative for portable or on-site continuous monitoring, with benefits of low cost, high reliability and validity, and ease of operation[16,17]. Structurally, ISEs can be categorized into liquid-contact (with an internal filling solution), and solid-contact (featuring an ion-to-electron transducer) configurations[18]. Regardless of the configuration, their core performance characteristics—sensitivity (slope), detection limit (LOD), selectivity, and linear range—are primarily determined by the composition of the ion-selective membrane (ISM). The ISM is typically a complex mixture composed of a polymer matrix (e.g., PVC), a plasticizer, an ionophore (ion carrier), and often an ionic additive (ion exchanger)[19]. The ionophore is responsible for selectively extracting target ions from the sample interface into the ISM. Therefore, ionophores typically possess functional groups capable of accommodating multiple target ions or structures with coordination spaces and specific binding sites that enable ion-selective recognition[20].
The selectivity of traditional ionophores, such as lipophilic quaternary ammonium salts, for various anions generally follows the Hofmeister series[21], as their electrostatic interactions with target anions govern the observed potential responses. This trend, for anions, is typically observed as: ClO4− > SCN− > I− > salicylate > NO3− > Br− > NO2− > Cl− > HSO3− > acetate > SO42− > HPO42−. Ionophore selectivity can be enhanced and tailored during synthesis to render the ligand site more sterically compatible with perchlorate ions[22]. Numerous studies have evaluated the application of newly synthesized ionophores derived from various structures, including calix(4)arenes[23−26], metal complexes (Ni[27−29], Pt[30,31], Co[32,33], Cu[34,35], Zn[36], Au[37], etc.) porphyrins[38,39] and other supramolecular architectures[40−44]. Metalloporphyrin-based anion-selective electrodes frequently demonstrate anti-Hofmeister selectivity. Porphyrins and their metal complexes offer notable advantages as molecular recognition elements: they exist in diverse structural forms, feature a rigid molecular scaffold, allow precise control over ring substituent positioning, and enable modulation of both the steric environment around the axial coordination site and interaction directionality via variation of the central metal ion[45]. Therefore, further investigation is warranted into how substituent structures at specific ring positions influence the hydrophilic/hydrophobic properties of porphyrins, as well as the role of different coordinated metal ions.
In this study, a plasticized PVC membrane perchlorate-selective liquid electrode was developed, utilizing metal-based porphyrin derivatives as ionophores. This electrode offers a wide linear dynamic range and robust anti-interference capability, making it suitable for simple and rapid perchlorate determination. The developed electrode has been successfully used for the direct measurement of wastewater from fireworks and surface water, eliminating the need for pretreatment of the water samples.
-
Unless otherwise noted, reagent grade chemicals were purchased and used as received. The chemical reagents used as ionophores included the following metalloporphyrins: iron(III) meso-tetraphenylporphine chloride (Fe[III]TPPCl), iron(III) 5,10,15,20-tetrakis(4-methylphenyl)porphyrin chloride (Fe[III]TMPPCl), iron(III) 5,10,15,20-tetrakis(4-chlorophenyl)porphyrin chloride (Fe[III]TClPPCl), cobalt(II) tetraphenylporphyrin (Co[II]TPP), copper(II) tetraphenylporphyrin (Cu[II]TPP), manganese(III) meso-tetraphenylporphine chloride (Mn[III]TPPCl), and zinc(II) tetraphenylporphyrin (Zn[II]TPP). The following ion-pair agents were employed as the ionic additives, including hexadecyl trimethyl ammonium bromide (CTAB), tridodecylmethylammonium chloride (TDMACl), and methyltrioctylammonium chloride (MTOACl). The reagents used as plasticizers were bis(2-ethylhexyl) sebacate (DOS), Dibutyl phthalate (DBP), Dioctylphthalate (DOP), and ortho-nitrophenyloctyl ether (NPOE), and the polymer matrix was selected as high molecular weight Poly(vinyl chloride) (PVC). These chemicals are listed in Supplementary Table S1, along with chemical structure and CAS number.
Stock solutions (1 M) of ClO4− and interfering anions (e.g., PO43−, SO42−, CO32−, NO3−, Cl−) were prepared in deionized water (≥ 18 MΩ·cm), and working solutions were obtained by serial dilution as needed.
ISE preparation and optimization
-
The ISMs were prepared by dissolving specific amounts of PVC, plasticizer, ionophore, and ionic additive in 5 mL of tetrahydrofuran (THF). The homogeneous solution was poured into a glass culture dish (diameter ~35 mm), and covered loosely to allow THF evaporation overnight in an electric thermostatic drying oven, resulting in a smooth elastic membrane with thickness of ~0.8 mm. Small disks (φ ~7 mm) were punched using a hole puncher from the master membrane.
As designed in Supplementary Fig. S1, the ISE body was machined using a polyoxymethylene tube with an outer diameter of ~12 mm. A small disk of the prepared ISM was fixed to one end with a cap, and an Ag/AgCl wire was inserted and sealed in the other end. The ISE was filled with solution (10−2 M NaClO4·H2O), and conditioned in 10−2 M NaClO4·H2O solution for at least 24 h before use.
The experiments for the screening of ionophores, ionic additives, and plasticizers and the optimization of their composition are listed in Table 1. Key performance parameters, including linearity, sensitivity, and selectivity, were determined.
Table 1. Optimization of PVC-based membrane sensors with varied plasticizers and ionophores: comparative evaluation of sensitivity, linearity, and selectivity for metal-based porphyrin derivatives detection
Category Ionophores Plasticizers Ionic additives PVC / plasticizers / ionophores / ionic additives (mg)a Evaluation test I Fe(III)TPPCl NPOE CTAB 600 :1200 : 20 : 60 Linearity, Sensitivity and Selectivity Coefficient Fe(III)TMPPCl 600 : 1200 : 20 : 60 Fe(III)TClPPCl 600 : 1200 : 20 : 60 Co(II)TPP 600 : 1200 : 20 : 60 Cu(II)TPP 600 : 1200 : 20 : 60 Mn(III)TPPCl 600 : 1200 : 20 : 60 Zn(II)TPP 600 : 1200 : 20 : 60 II Fe(III)TPPCl DOS CTAB 600 : 600 : 20 : 60 Linearity and Sensitivity 600 : 900 : 20 : 60 600 : 1200 : 20 : 60 600 : 1500 : 20 : 60 DOP 600 : 600 : 20 : 60 600 : 900 : 20 : 60 600 : 1200 : 20 : 60 600 : 1500 : 20 : 60 DBP 600 : 600 : 20 : 60 600 : 900 : 20 : 60 600 : 1200 : 20 : 60 600 : 1500 : 20 : 60 NPOE 600 : 600 : 20 : 60 600 : 900 : 20 : 60 600 : 1200 : 20 : 60 600 : 1500 : 20 : 60 III Fe(III)TPPCl NPOE TDMACl 600 : 1200 : 20 : 10 Linearity, Sensitivity and Selectivity Coefficient 600 : 1200 : 20 : 20 600 : 1200 : 20 : 40 CTAB 600 : 1200 : 20 : 10 600 : 1200 : 20 : 20 600 : 1200 : 20 : 40 MTOACl 600 : 1200 : 20 : 10 600 : 1200 : 20 : 20 600 : 1200 : 20 : 40 a Add the components to 5 mL of THF in the listed proportions. Electromotive Force (EMF) measurements and ISE evaluation
-
All EMF measurements of ISEs were performed at room temperature (25 ± 1 °C) using a high-impedance digital mV meter/pH meter (Model PHSJ-6L, Leici, China) with an Ag/AgCl reference electrode (Model 216, Xianfeng, China). The EMF measurement of the ISE electrode was conducted with the working solutions of ClO4− concentration ranging from 10−7 to 10−1 M. When measuring, the working solution or water sample was magnetically stirred, and the potentiometric response was record after stabilization. The slope (mV·decade−1) and linear range were calculated from the calibration curve (E vs logc[ClO4−]), and the practical LOD was determined as the intersection of the extrapolated linear segments of the calibration curve.
The selectivity coefficients were determined using the separate solution method (SSM). Initially, potential responses were measured in the target ion solution, and Ei0 was calculated by extrapolating the response to ai = 1 M. Subsequently, potentiometric responses were measured for various interfering ions. Ej0 was determined using the same procedure. Finally, the selectivity coefficients (Kij, where i is the target ion, and j is the interfering ion) were calculated using the potential at 1 M based on the Nicolsky-Eisenman equation[46]:
$ {K_{ij}} = \exp \left\{ \dfrac{{E_j^0 - E_i^0}}{{RT}}{z_i}F \right\} $ (1) where, zi is the charge of the target ion. R, T, and F denote the gas constant, the absolute temperature, and the Faraday constant, respectively.
The dynamic response time is a critical performance parameter for perchlorate-selective electrodes, as it determines the electrode's applicability for real-time monitoring scenarios[47]. In this study, the response behavior of the optimized Fe(III)TPPCl-based electrode was evaluated under stepwise increases in ClO4− concentration, ranging from 1.0 × 10−5 to 1.0 × 10−1 M. The pH of the sample solution is a critical factor that can significantly influence the response of the ISE. Thus, the influence of pH was investigated by recording the potential of 10−4 M to 10−1 M ClO4− solutions while adjusting the pH from 2 to 10 using incremental additions of 1 M H2SO4 or NaOH.
The prepared ISE was finally applied to 15 surface water and five firework manufacturing wastewaters, which were spiked with 100 and 200 mg·L−1 of ClO4−, respectively.
-
The selection of suitable ionophores is fundamental to the design of ISEs, as it directly determines their selectivity, sensitivity, and detection performance toward target ions[48]. To ensure consistency across formulations, all membranes were fabricated using a fixed composition of PVC (32 wt%, 600 mg), plasticizer (NPOE, 64 wt%, 1,200 mg), CTAB, and candidate ionophore at a 3:1 weight ratio. The performances of ISEs using these porphyrin-based ionophores were systematically compared based on three key parameters, including response slope, LOD, and selectivity toward ClO4− vs common interfering anions (Fig. 1, Supplementary Table S2).
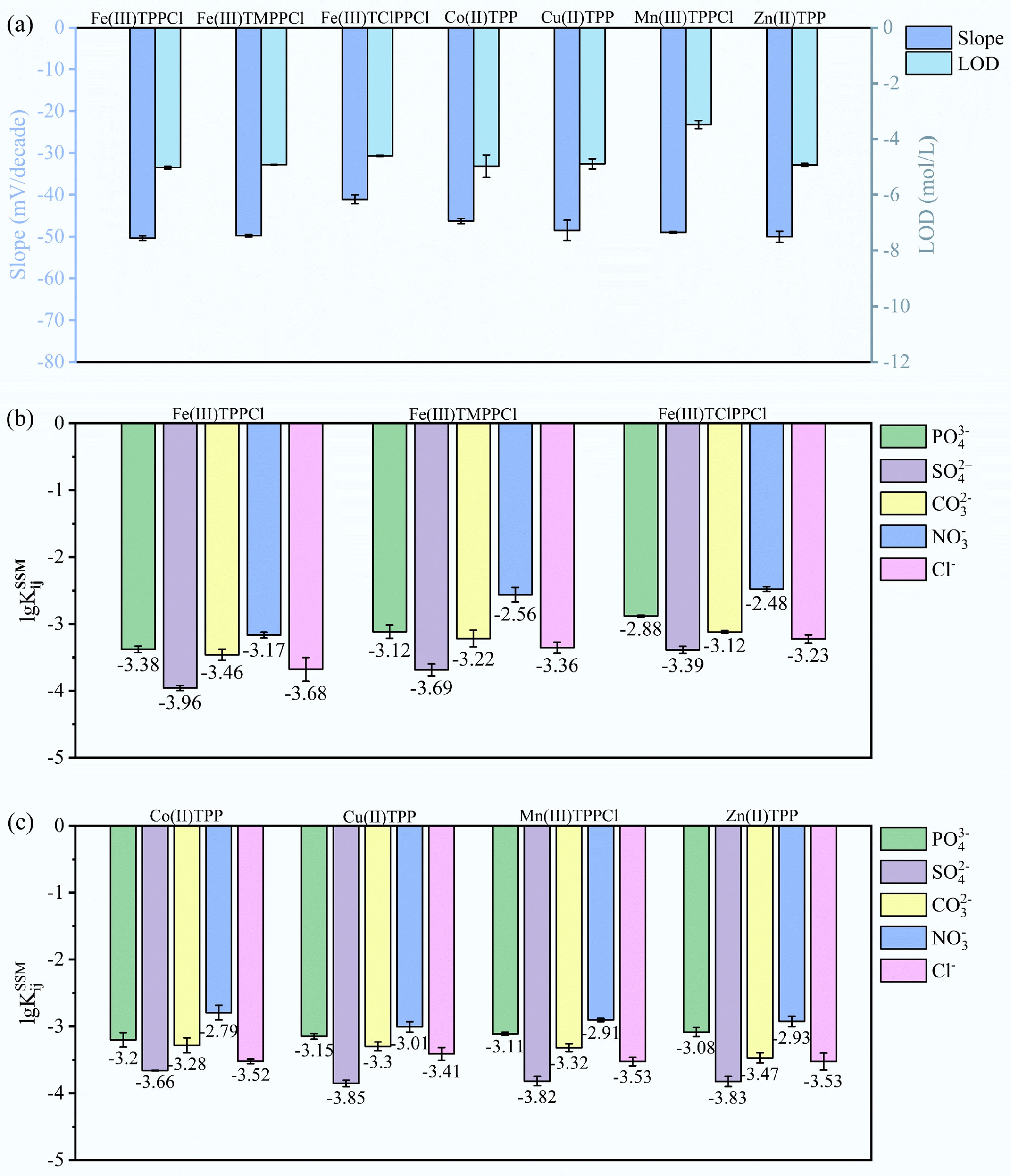
Figure 1.
Response slopes, detection limits, and selectivity coefficients for porphyrin-based ionophores. (a) Response slopes (left axis) and detection limits (right axis). (b) Selectivity coefficients logKijSSM of porphyrin-based ionophores with different substituents. (c) Selectivity coefficients logKijSSM of porphyrin-based ionophores with different coordinated metal ions.
As shown in Fig. 1a, the left and right axes represent the response slope and LOD of ISEs, respectively. Among the porphyrin-based ionophores with different substituent structures, Fe(III)TPPCl exhibited the most favorable response, with a response slope −50.37 ± 0.56 mV·decade−1. In contrast, Fe(III)TClPPCl exhibited a comparatively lower slope (−41.08 ± 1.05 mV·decade−1), suggesting suboptimal ion recognition. Thus, the porphyrin substituted with electron-withdrawing group would decrease the response slope of ISE. For the porphyrin-based ionophores with different coordinated metal ions, Fe(III)TPPCl, Cu(II)TPP, and Zn(II)TPP exhibited comparable response slope with LODs reached ~10−5 M; while the Co(II)TPP showed an observable lower response slope (−46.29 ± 0.62 mV·decade−1), and Mn(III)TPPCl exhibited a poor LOD of ~10−3.5 M.
Figure 1b, c compared the logarithmic selectivity coefficients (logKijSSM) of ISEs, using porphyrin-based ionophores with different substituent groups and different coordinated metal ions, against a range of interfering anions including PO43−, SO42−, CO32−, NO3−, and Cl−. In general, the selectivity of all the ISEs against interfering ions followed the order as follows: SO42− > Cl− > CO32− > PO43− > NO3−. Among porphyrin-based ionophores with different porphyrin ring structures, the Fe(III)TPPCl exhibited the strongest selectivity, particularly against SO42− (logKijSSM = –3.96), indicating a strong preference for sulfate over other competing anions, while the Fe(III)TClPPCl showed the lowest selectivity; its porphyrin ring is substituted with electron-withdrawing group. Among the test coordinated metal ions, the selectivity slightly decreased in the order of Fe(III)TPPCl > Cu(II)TPP > Zn(II)TPP > Mn(III)TPPCl > Co(II)TPP. The superior performance of Fe(III)TPPCl toward ClO4− is attributed to the preferential axial coordination between Fe(III), a hard Lewis acid, and ClO4−, which is classified as a hard Lewis base due to its high electronegativity, low polarizability, and strongly hydrated tetrahedral structure composed of oxygen atoms[49−51]. This hard-hard acid-base pairing favors specific electrostatic interactions, contributing to selective perchlorate recognition over softer or more polarizable anions. Additionally, the binding constant of this perchlorate interaction is significantly higher than that of most other anions.
Consequently, Fe(III)TPPCl was screened as the most effective ionophore towards ClO4−, offering the optimal combination of sensitivity, low LOD, and high selectivity. However, its slope did not reach the theoretical Nernstian value of –59 mV·decade−1, and thus further optimization of ISM composition is required to enhance its overall electrode performance in practical applications.
Selection and optimization of ISM plasticizer
-
Plasticizers, such as DOS, DOP, DBP, and NPOE, are incorporated into ISMs to enhance mechanical flexibility, facilitate ion mobility, and regulate membrane dielectric properties. Selecting a plasticizer with appropriate polarity ensures compatibility with the ionophore and further promotes enhanced selectivity and sensitivity of the membrane[20,52]. As shown in Table 2 and Supplementary Table S3, a variety of plasticizer types and loadings were tested for the ISMs with the constant amounts of polymer matrix, ionophore, and ionic additives (i.e., 600 mg PVC, 20 mg Fe[III]TPPCl, and 60 mg CTAB). With increasing plasticizer proportion, the performance of ISMs in terms of linear range and LOD initially demonstrated enhancement, followed by marginal performance deterioration. Consistent with previous literature[53], the optimal ratio of plasticizer to PVC is 2:1–2.5:1. Under this proportional condition, the ISM using NPOE achieved a slope of –44.03 mV·decade−1, an extended linear range from 10−7 to 10−1 M, and a remarkably LOD of 10−6.53 M, which is below the WHO limit and median LOD of the reported ISEs[22]. This is attributed to NPOE's high dielectric constant (~23.1), which promotes ion migration and improves the ion-exchange equilibrium at the membrane interface[54]. For the other plasticizers, the corresponding detection limits ranged from 10−5.04 to 10−5.1 M. Additionally, the response slopes of the ISMs were observed in an order of NPOE > DBP > DOS > DOP.
Table 2. Composition of membranes and potentiometric response characteristics of perchlorate-selective electrodes based on Fe(III)TPPCl
No. Composition (mg) Slope (mV·decade−1) Linear range (M) LOD (M) PVC Plasticizers Ionophores Ionic additives 1 600 DOS, 600 Fe(III)TPPCl, 20 CTAB, 60 −46.33 10−4 ~ 10−1 10−4.07 DOS, 900 −39.89 10−5 ~ 10−1 10−5.17 DOS, 1200 −47.78 10−5 ~ 10−1 10−5.07 DOS, 1500 −40.48 10−5 ~ 10−1 10−4.95 2 600 DOP, 600 Fe(III)TPPCl, 20 CTAB, 60 −31.65 10−4 ~ 10−1 10−3.99 DOP, 900 −35.48 10−4 ~ 10−1 10−3.9 DOP, 1200 −39.05 10−5 ~ 10−1 10−5.04 DOP, 1500 −35.15 10−5 ~ 10−1 10−4.95 3 600 DBP, 600 Fe(III)TPPCl, 20 CTAB, 60 −40.66 10−4 ~ 10−1 10−4.01 DBP, 900 −43.09 10−5 ~ 10−1 10−5.21 DBP, 1200 −50.83 10−5 ~ 10−1 10−4.99 DBP, 1500 −55.09 10−5 ~ 10−1 10−5.1 4 600 NPOE, 600 Fe(III)TPPCl, 20 CTAB, 60 −50.92 10−3 ~ 10−1 10−3.17 NPOE, 900 −57.08 10−3 ~ 10−1 10−3.47 NPOE, 1200 −44.03 10−7/10−6 ~ 10−1 10−6.53 NPOE, 1500 −59.19 10−4 ~ 10−1 10−4.3 Thus, the plasticizer and polymer matrix composition for ISM based on Fe(III)TPPCl ionophore was optimized as NPOE at a 2:1 ratio to PVC.
Selection and optimization of ISM ionic additive
-
Ionic additives play a crucial role in ion-selective membranes by facilitating specific ion transport while excluding interferents. They also neutralize the charge of the ionophore-analyte complex, thereby enhancing membrane conductivity and enabling efficient ion exchange[55]. Figure 2 and Supplementary Table S4 systematically compare the effect of ionic additives on the sensitivity, LOD, and selectivity of ISMs, which were fabricated using the optimal ionophore Fe(III)TPPCl and plasticizer NPOE (NPOE : PVC = 2:1), while varying the type and concentration of ionic additives (TDMACl, CTAB, MTOACl) at weight ratios of 1:2, 1:1, and 2:1 relative to the ionophore.
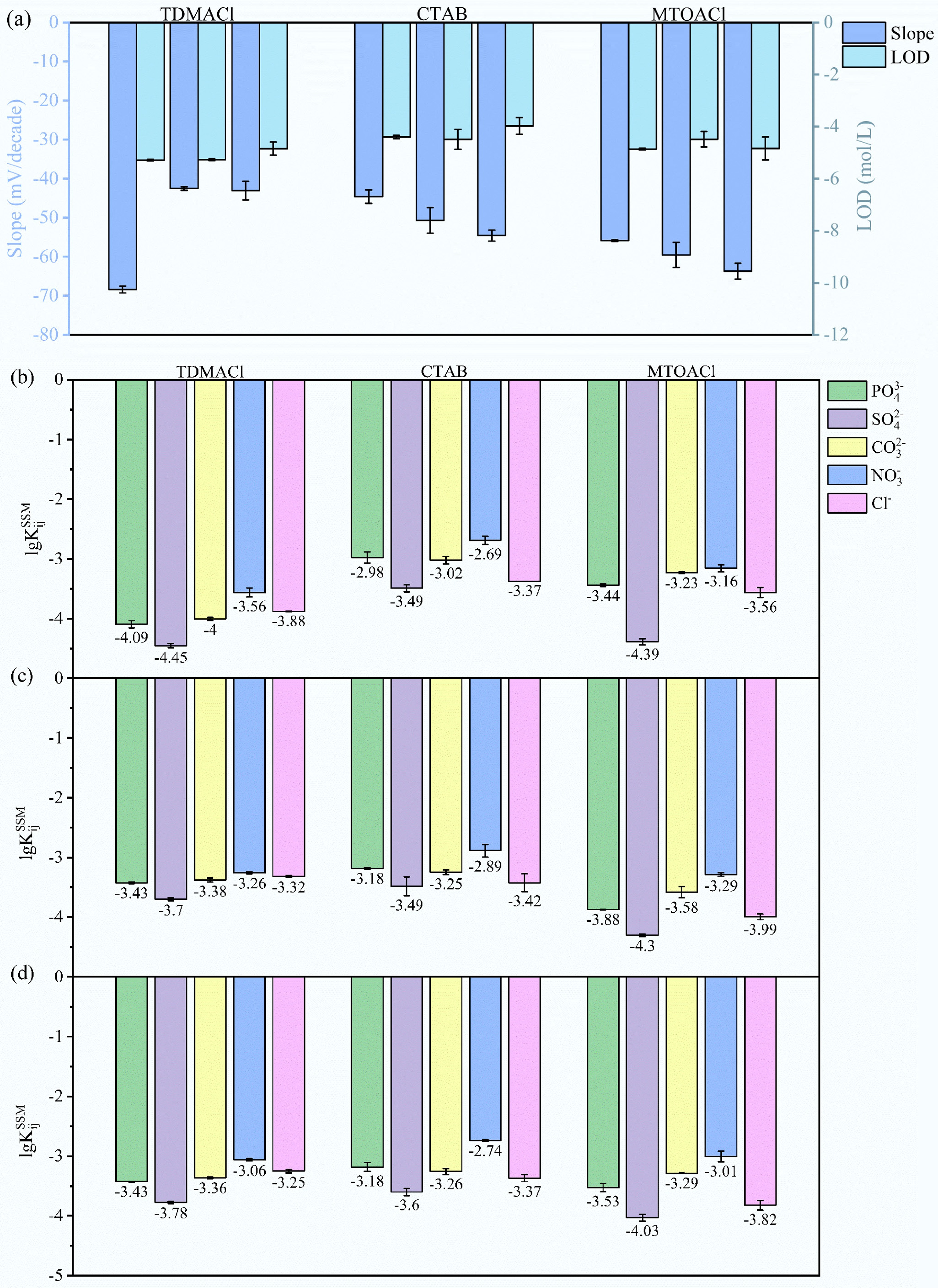
Figure 2.
Response slopes, detection limits, and selectivity coefficients for ionic additives at varying additive-to-ionophore ratios. (a) Response slopes (left axis) and detection limits (right axis) of various ionic additives at 1:2, 1:1, 2:1 additive-to-ionophore ratios. (b) Selectivity coefficients logKijSSM of ionic additives at a 1:2 additive-to-ionophore ratio against various interfering ions. (c) Selectivity coefficients logKijSSM of ionic additives at a 1:1 additive-to-ionophore ratio against various interfering ions. (d) Selectivity coefficients logKijSSM of ionic additives at a 2:1 additive-to-ionophore ratio against various interfering ions.
As shown in Fig. 2a, the left Y-axis indicates the slope (mV·decade−1) of the electrode response to ClO4− under various ionic additive conditions. Among all tested formulations, the membrane containing TDMACl at a 1:2 ratio to Fe(III)TPPCl exhibited the highest sensitivity with a slope of –68.42 ± 0.91 mV·decade–1, exceeding the theoretical Nernstian value, suggesting the addition of specific amounts of ionic additives enhances the sensitivity of the electrode. For TDMACl, increasing its proportion beyond the 1:2 ratio led to reduced slopes, suggesting that excess TDMACl compromises membrane performance. In contrast, CTAB and MTOACl showed increasing slopes with additive concentration, with MTOACl reaching near-Nernstian performance at –59.71 ± 3.89 mV·decade−1, whereas CTAB remained suboptimal in sensitivity, with response slopes below the theoretical Nernstian value. The right Y-axis in Fig. 2a illustrates the LODs, with lower values indicating higher sensitivity. Both TDMACl and MTOACl achieved low LODs around 10−5 M, highlighting their superior detection capabilities. In contrast, CTAB resulted in relatively poorer LODs, suggesting less favorable ion exchange efficiency and membrane conductivity.
Figure 2b–d further presents the selectivity of ISM to ClO4−, against interfering anions PO43−, SO42−, CO32−, NO3−, and Cl−. Notably, the TDMACl : Fe(III)TPPCl = 1:2 configuration yielded the most favorable selectivity profile, with logKijSSM values lower than –4 against SO42−, PO43−, and CO32−. The selectivity coefficients obtained for the proposed electrode showed a selectivity sequence of anions in the following order: SO42−> PO43− > CO32− > Cl− > NO3−. This superior performance is likely attributed to the high lipophilicity of TDMACl, which enhances membrane conductivity, facilitates rapid ion exchange, and improves ClO4− affinity while reducing resistance and interference. Moreover, lipophilic additives promote phase homogeneity and prevent ion-pair formation with interfering anions, thereby increasing the selectivity and stability of the membrane[34,56]. The final optimized membrane composition was determined to be: Fe(III)TPPCl (1.1 wt%), NPOE (65.6 wt%), PVC (32.8 wt%), and TDMACl (0.5 wt%).
Performances of the optimized ISE
-
The dynamic response time is a critical performance parameter for ISE, as it determines the electrode's applicability for real-time monitoring scenarios. Figure 3a presents the time-resolved potential response of the optimized ISE upon successive ClO4− concentration steps from 1.0 × 10−5 to 1.0 × 10−1 M. Notably, the electrode achieved a stable potential within approximately 5 s after each concentration change, confirming its rapid response characteristics. The clear and stable plateaus corresponding to each concentration level indicated a well-defined Nernstian behavior. The optimized ISE also showed a wide linear range from 10−5 to 10−1 M with a slope of –66.63 ± 1.29 mV·decade−1 and a coefficient of determination R2 = 0.999, as shown in the inset. For detailed data, refer to Supplementary Table S5. The electrode stability test was conducted in a 10−4 M NaClO4·H2O solution over a continuous 5-h period. The potential fluctuated within a narrow range of 297 to 305 mV, indicating good short-term stability. For detailed data, refer to Supplementary Table S6 and Supplementary Fig. S2.
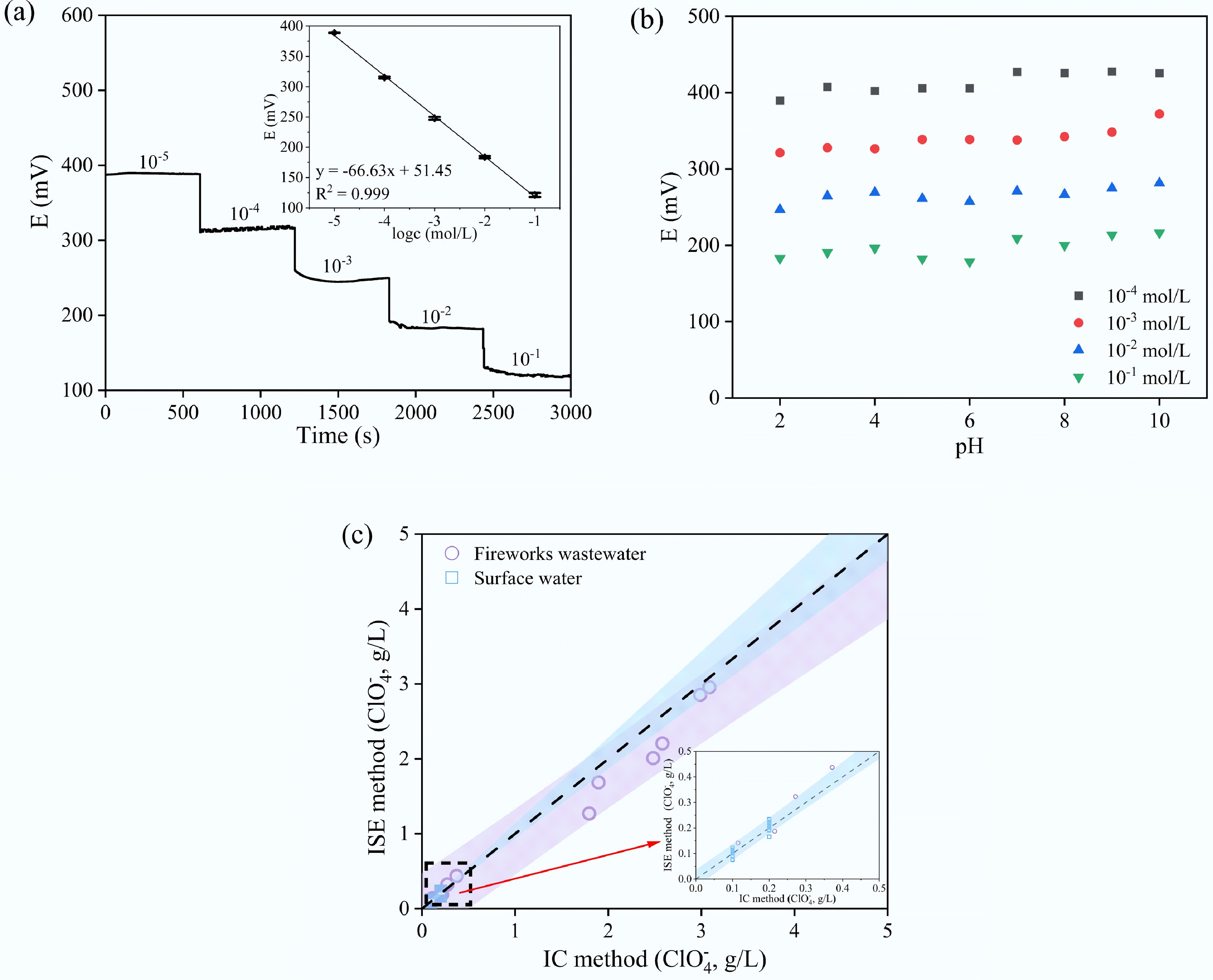
Figure 3.
Evaluation performance of the proposed ISE. (a) Dynamic response time of optimal electrode for step changes in concentration of ClO4− (low to high), inset graph represents the linear region of the average values obtained over the 2- to 10-min interval. (b) Effect of pH of test solutions on the response of the proposed optimized electrode: 10−4–10−1 M NaClO4·H2O. (c) Evaluation of ion-selective electrode accuracy in real wastewater samples.
Figure 3b depicts the pH dependence of the optimized ISE over the pH range from 2 to 10 at a constant ClO4− concentration of 10−4, 10−3, 10−2, and 10−1 M, respectively. The potentiometric response of ISE remained relatively stable at an acidic pH no matter of the ClO4− concentration, suggesting that the competitive protonation or deprotonation of the ion carrier or plasticizer was not significantly affected[57,58]. The potentiometric response slightly increased when pH increased to ~10, which is possibly due to interference of hydroxide ions for ClO4− exchange sites on the membrane. Despite being slightly affected by alkaline pH, the optimized ISE exhibited a mean response slope of –72.22 ± 2.83 mV·decade−1 and a R2 value of 0.997 ± 0.003 across all pH conditions (Supplementary Table S7).
To validate its practical applicability, the optimized ISE was employed to quantify ClO4− concentrations in 15 surface water and five firework manufacturing wastewater samples, which were further spiked with 100 and 200 mg·L−1 ClO4−, respectively. As summarized in Supplementary Table S8, the original ClO4− concentrations in surface water samples were negligible, and the recovery rates were in the range of 75.9%–123.1% with an average value of 104.3%; while the original ClO4− concentrations fireworks manufacturing wastewater samples were 14.8, 171.8, 1,695.2, 2,381.9, and 2,886.5 mg·L−1, respectively, and the recovery rates ranged from 70.8% to 122.9% with a mean value of 96.4%. As illustrated in Fig. 3c, a strong correlation was observed between perchlorate concentrations measured by the ion-selective electrode (ISE) method and ion chromatography (IC) in firework wastewater (purple circles) and surface water (blue squares). The shaded regions represent the 95% confidence intervals for each data set, and the inset highlights the low concentration range (0–500 mg·L−1), illustrating good agreement between the two methods across different sample matrices. These findings confirm that the proposed electrode is not only selective and sensitive but also robust for in situ determination of perchlorate in diverse environmental water bodies.
-
This study successfully developed and optimized a high-performance perchlorate-selective electrode based on Fe(III)TPPCl as the ionophore. Key findings include ionophore screening, which confirmed Fe(III)TPPCl's superiority over other metalloporphyrins (e.g., Co[II]TPP, Mn[III]TPPCl) and substituted analogs (e.g., Fe[III]TClPPCl), delivering optimal sensitivity (slope: –68.42 ± 0.91 mV·decade−1), low LOD (10−5 M), and exceptional selectivity against common anions (SO42− > PO43− > CO32− > Cl− > NO3−). The Fe(III) center's axial coordination with ClO4− and electron-donating phenyl groups were critical in promoting anti-Hofmeister behavior. Membrane optimization identified NPOE as the ideal plasticizer (dielectric constant ~23.1) at a 2:1 ratio to PVC, extending the linear range to 10−5–10−1 M. TDMACl enhanced sensitivity and selectivity by improving ion exchange kinetics and membrane homogeneity. Real-world applicability was demonstrated in fireworks wastewater (perchlorate: 14.8–2,886.5 mg·L−1) and surface water. The electrode achieved quantitative recoveries (96.4% mean for wastewater and 104.3% for surface water) without the need for sample pretreatment, closely aligning with ion chromatography results. Its robustness across pH 2–10 and rapid response (< 5 s) support its field deployment. Plus, based on current raw material pricing and fabrication procedures, the estimated cost per unit for the proposed liquid-contact ISE is below USD
${\$} $ Despite its favorable performance, this study is subject to certain limitations. The current electrode design relies on a liquid-contact configuration, which may present challenges for long-term deployment due to potential leakage of the internal solution or instability under varying temperatures. Future work will focus on prolonging electrode lifespan and transitioning to solid-contact ISE configurations to improve field robustness.
-
It accompanies online at: https://doi.org/10.48130/een-0025-0007.
-
All authors contributed to the study conception and design. Data collection and analysis were performed by Baichun Li, Bing Li, Chengkui Liang, and Shuo Zhang. Conceptualization and the first draft of the manuscript was written by Baichun Li, and all authors commented on previous versions of the manuscript. Funding acquisition was provided by Wentao Li, and resources were provided by Qimeng Li. Methodology development and validation were led by Yuze Han, with supervision and project administration by Wentao Li. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed in the current study are available from the corresponding author on reasonable request.
-
This work was supported by the National Key Research and Development Program of China (2022YFC3204900).
-
Wentao Li is an editorial board member of Energy & Environment Nexus and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
-
Porphyrin-based derivatives with diverse substituents and coordinated metal ions were evaluated as ClO4− ionophores.
The optimized ISM composition consists of 1.1 wt% Fe(III)TPPCl, 65.6 wt% NPOE, 32.8 wt% PVC, and 0.5 wt% TDMACl.
The proposed ISE exhibits a high response slope, low LOD, excellent selectivity, and a wide linear detection range.
Rapid response (< 5 s) and broad pH stability (2–10) enable robust perchlorate detection in diverse environments.
The ISE shows high accuracy in sample analysis, making it suitable for monitoring perchlorate in fireworks wastewater.
-
Full list of author information is available at the end of the article.
- Supplementary Table S1 Comprehensive list of functional materials and chemicals with CAS Registry Numbers and structure.
- Supplementary Table S2 Response slope, LOD, and selectivity toward ClO4− versus common interfering anions.
- Supplementary Table S3 Response slope and LOD with different plasticizers and ratios.
- Supplementary Table S4 Response slope, LOD, and selectivity toward ClO4− versus common interfering anions with different ionic additives and ratios.
- Supplementary Table S5 The potential was continuously measured over a 10-minute period across a concentration gradient ranging from 10−5 to 10−1 M.
- Supplementary Table S6 The electrode stability continuously monitored over a 5-hour period in a 10−4 M solution of NaClO4·H2O.
- Supplementary Table S7 Response slope and R2 of proposed electrode under the condition of various concentrations and pH.
- Supplementary Table S8 Determination of perchlorate in spiked samples by using proposed perchlorate-selective electrodes.
- Supplementary Table S9 Estimated material costs for one unit of optimized ion-selective membrane.
- Supplementary Fig. S1 Schematic diagram of the ion-selective electrode structure.
- Supplementary Fig. S2 The electrode stability was continuously monitored over a 5-hour period in a 10−4 M solution of NaClO4·H2O.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li B, Li B, Han Y, Li Q, Liang C, et al. 2025. Rational design of porphyrin-based ionophores for enhanced perchlorate selectivity in ion selective electrodes: application to fireworks wastewater analysis. Energy & Environment Nexus 1: e009 doi: 10.48130/een-0025-0007
Rational design of porphyrin-based ionophores for enhanced perchlorate selectivity in ion selective electrodes: application to fireworks wastewater analysis
- Received: 30 June 2025
- Revised: 21 July 2025
- Accepted: 22 August 2025
- Published online: 20 October 2025
Abstract: The high-concentration perchlorate (ClO4−) wastewater discharged by the firework and firecracker industry poses a significant threat to the ecological security of the receiving water bodies and drinking water sources. In the context of the Internet of Things (IoT), monitoring high-concentration perchlorate wastewater necessitates intelligent control and management. This study introduces a liquid-contact ion-selective electrode (ISE) featuring novel porphyrin-based ionophores for the rapid, sensitive, and selective determination of perchlorate determination. The electrode features a poly(vinyl chloride) (PVC)-based membrane, typically called an ion-selective membrane (ISM), optimized with iron(III) meso-tetraphenylporphine chloride (Fe[III]TPPCl) as the ionophore, ortho-nitrophenyloctyl ether (NPOE) as the plasticizer, and tridodecylmethylammonium chloride (TDMACl) as the ionic additive. Systematic screening revealed Fe(III)TPPCl's superior performance over other metalloporphyrins, exhibiting a near-Nernstian slope (–68.42 ± 0.91 mV·decade–1), a low detection limit (LOD = 10−5 M), and strong anti-interference capability, particularly against sulfate (logKijSSM = –4.45). Optimization of membrane composition (1.1 wt% Fe[III]TPPCl, 65.6 wt% NPOE, 32.8 wt% PVC, 0.5 wt% TDMACl) enabled a wide linear range (10−5–10−1 M), fast response time (< 5 s), and stability across pH 2–10. The ISE was validated for real-sample analysis, achieving a mean recovery of 104.3% in spiked surface water and 96.4% in firework wastewater without pretreatment. Results correlated well with ion chromatography, confirming the electrode's accuracy. This work demonstrates a cost-effective, field-deployable sensor for ClO4− monitoring in complex environmental matrices, addressing critical regulatory compliance needs.