-
Oral Dissolving Film (ODF), also known as Tongue Film (TF), is a thin, postage stamp-shaped film with a surface area of 5–20 cm2, and a mass not exceeding 0.2 g. It rapidly dissolves in the oral cavity. The United States Pharmacopeia defines a film as a thin sheet placed in the mouth, potentially consisting of multiple layers, and specifies the site of drug release from the film[1].
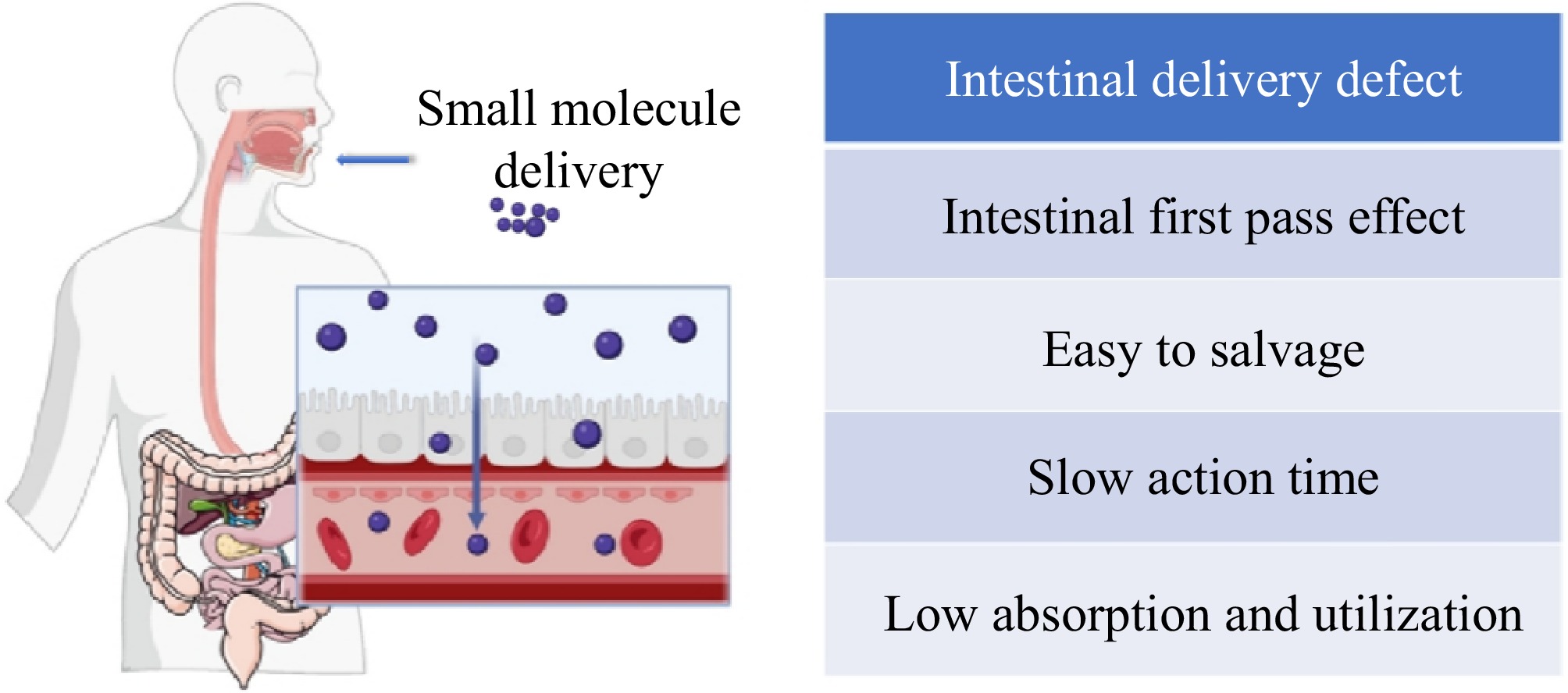
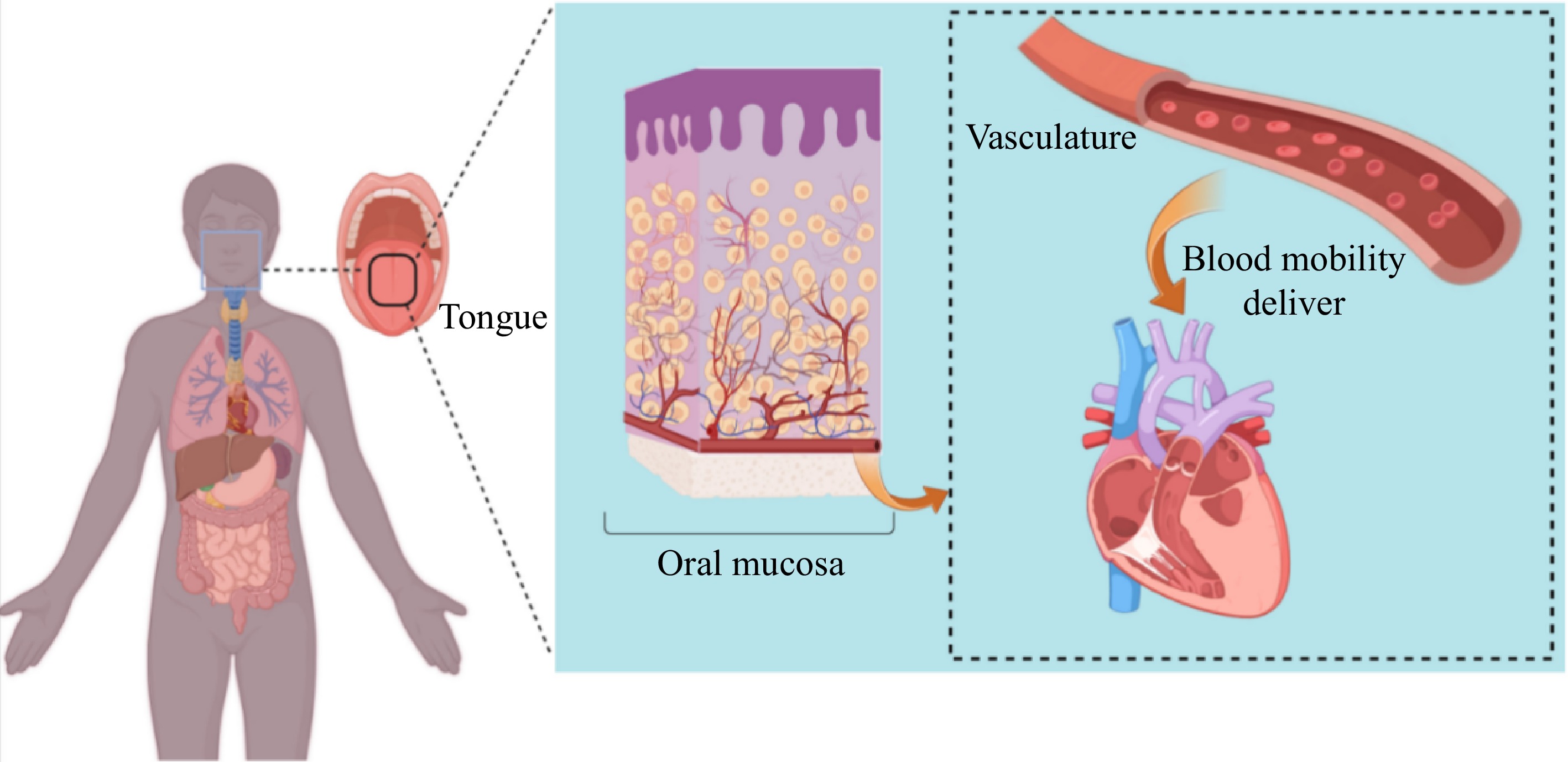
The ODF classifies membranes into three categories: monolayers, extended release membranes, and buccal dispersible membranes that dissolve in water. The selection of the appropriate membrane type is based on the characteristics of the encapsulated substance. Buccal and sublingual films are designed to be absorbed through the mucosa in the mouth, allowing for bypassing of first-pass metabolism and gastrointestinal degradation of the substance. Through the buccal mucosa, substances can enter the blood circulatory system via two main pathways: a cellular bypass pathway and an intracellular pathway. The intracellular pathway is the primary route for most substances to enter the cell. The buccal mucosa has a large surface area and good osmotic effect, so oral delivery is now considered to be the most effective part of the systemic delivery, and the delivered substances can be released and absorbed in the oral cavity, which greatly improves the bioavailability of the substances[2]. The buccal mucosa, with its extensive surface area and strong osmotic effect, is now recognized as the most effective route for systemic delivery. Substances delivered orally can be released and absorbed in the oral cavity, significantly enhancing their bioavailability[3]. The emergence of ODF breaks the inherent dosage form that can only be delivered by solid tablets, granules, capsules, or liquids, and it is a new delivery method that is at the forefront of the food industry. It is a new type of delivery method in the food industry. According to the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in the United States, most of the nutrients needed by the human body are obtained from food. In 2023, the IHME reported that the global issue of malnutrition is affecting nearly a third of the world's population. The IHME emphasized the urgent need to enhance food policies and promote awareness of healthy eating habits. Nutritional supplementation is currently a popular trend in society, with the majority of available supplements on the market being in the form of liquid, solid chewable tablets, or capsules. These products may provide nutritional supplementation, but can be inconvenient to consume. For example, liquid nutritional elements have a shorter gastric emptying time[4], limiting nutrient absorption. Tablets require a large amount of water for ingestion, increasing the risk of throat obstruction or choking, most of the capsules have a special odor, leading to poor compliance. As a result, the content of small molecules is reduced and cannot be fully utilized. Infants and young children do not yet have good chewing ability and do not have a sense of autonomy. When taking supplements, one may be at risk of choking. This is especially true for middle-aged individuals with ageing teeth that are becoming loose, causing a decline in chewing function. As a result, many elderly people or patients may be hesitant to take supplements due to swallowing disorders, which can lead to choking on food and malnutrition. This can ultimately result in physical decline[5]. To solve the problem of dysphagia, orally disintegrating tablets were introduced (Table 1). However, these tablets cannot be rapidly released in a short period of time. Due to the high likelihood of accidental ingestion in the mouth, orally disintegrating tablets may be easily damaged during processing, transportation, and storage. Therefore, it is essential to develop a more effective oral delivery form to address the challenges of swallowing difficulties and potential hazards[6].
Type Immediate release Adhesive type Slow release adhesion type Area (cm2) 2–8 2–7 2–4 Quality (g) 0.02–0.2 0.02–0.2 0.02–0.2 Thickness (μm) 10–70 50–500 50–250 Structure Monolayer Single/multilayer Multilayer Ingredients Water-soluble polymer Water-soluble polymer Low solubility polymer Part used Tongue Buccula Other parts of mouth Releasing time 60 s A few minutes Maximum 8–10 h Take effect Local/whole body Local/whole body Local/whole body ODF is released rapidly in the oral cavity and can be melted under the tongue, on the tongue, or buccal. The method of administration is simple and does not require a large amount of water. It also does not cause accidental swallowing[10]. Oral film tablets are small in size and volume, making them easy to carry. They also allow for precise control over the content of the encapsulated substances. While the packaging of oral film tablets can provide additional protection for the enclosed substances, it is important to note that some natural active molecules may degrade under certain conditions. For example, floridoside can degrade significantly under neutral intestinal pH conditions, and curcumin, being a lipophilic and hydrophobic substance, may not be well absorbed by the human body due to its inherent limitations (Fig. 1). There are also certain nutrients that are protected due to the different properties of the encapsulated substances. This is because they have a naturally low content in supplements and cannot be replenished in a timely manner in daily life (Fig. 2). Nowadays, oral instant film tablets are being used on a large scale in all kinds of medicines, and they are gradually gaining importance in the food industry. The oral membrane is rich in DHA, lactoferrin, VD3, calcium, iron, zinc, and other nutrients. This helps address the issue of young infants and children not being able to swallow nutrient supplements, thus compensating for their inability to do so[11]. This product makes up for the lack of swallowing ability of young infants and children who are unable to supplement their nutritional elements, and has been widely welcomed. BY-HEALTH's Flash Sleep Tablet contains 3 mg of melatonin per tablet, which melts in the mouth and can improve sleep. Oral instant film is made of polysaccharide as the main raw material, with appropriate excipients, and finally made into a film by the appropriate method[12]. Natural polysaccharides are non-toxic and have good adhesion, which extends the residence time of the film agent in the oral cavity and increases the amount of release. In the production of film solution, a crosslinking agent can be added to enhance its stability[13]. A crosslinking agent, also known as a curing agent, hardening agent, or ripening agent, serves the purpose of creating a three-dimensional mesh structure from the macromolecule. This improves properties such as strength, tensile strength, heat resistance, and solubility. A flavor correction agent can improve the taste of the film agent and cover up the unique flavor of certain substances. Commonly used flavor correction agents include citric acid, sucrose, xylitol, sodium saccharin, and other flavors and fragrances. In this paper, the main film-forming materials and auxiliary materials of oral instant film tablets, the preparation method of oral instant film tablets, and the subsequent determination of the indicators of the film tablets are described in detail.
The main film-forming materials of ODF are polysaccharides and their derivatives or non-toxic polymers. The polysaccharides used should be non-toxic, water-soluble, and stable after the formation of the film in order to achieve sustained release of substances[14]. Ideal film-forming materials should have a uniform texture, no special flavor, maintain long-term stability, no chemical reaction with the encapsulated substance, good ductility, and will not change the physicochemical properties with the extension of time. The type of film-forming material determines the appearance, flexibility, and preservation in transportation of the resulting film agent[15]. Renata utilized pregelatinized starch and hydroxypropyl methylcellulose as the primary raw materials, experimenting with various ratios to determine that the 20/80 ratio yielded the most favorable taste in the oral cavity[16]. On the other hand, Shin employed hyaluronic acid as the key raw material and incorporated vitamin C and catechol to create an antioxidant suitable for easy transportation and use in food products. It is important to note that different film-forming materials possess distinct physicochemical properties, with several common options elaborated upon below.
Polyvinyl alcohol
-
Polyvinyl alcohol (PVA) is a polymer with the chemical formula (C2H4O)n, which is made from vinyl acetate through a polymerization reaction and alcoholysis. It has a mostly white solid powder or flocculent appearance, and is non-toxic and odorless[17]. PVA is almost unaffected by weak acids, weak bases, and less restrictive storage conditions. Storage in aqueous solution will not deteriorate, and the preparation of the finished mixture of polyvinyl alcohol is transparent. PVA has good film-forming abilities, is biodegradable, and has good mechanical properties. It is also moderately priced. Therefore, PVA is widely used in chemical production and is also commonly used in the fields of materials, construction, food, textiles, and the beauty industry[18]. A well-formed loaded ginger nanofiber membrane was successfully prepared by the electrostatic spinning technique using polyvinyl pyrrolidone. PVA is the material of choice for membrane production due to its stable nature. The membrane agent produced is in good shape and is not easy to crumple[19]. Mirzaeei et al. successfully prepared an oral film containing doxycycline hydrochloride for the treatment of periodontitis using PVA, PCL, and PS as raw materials. This film allows for sustained release of the antibiotic in the oral cavity. The prepared membrane has good stability, mechanical properties, and drug-carrying capabilities, and it has a significant inhibitory effect on bacteria in periodontitis[20].
Pullulanose
-
Pullulan sugar, also known as Pullulan, is a natural macromolecular compound with the chemical formula (C37H62O30) n. It is an extracellular water-soluble neutral polysaccharide, appearing as a white solid powder that is odorless and tasteless[21]. Pullulan polysaccharide was discovered in 1938 in the fermentation broth of bud short pedunculated mycobacteria. It was successfully extracted from the medium of bud short pedunculated enzyme in 1958. It is easily soluble in water and has good film-forming properties. The successfully prepared film agent is transparent in color, non-toxic, odorless, ductile, and stable[22]. Pullulan is used in the production of ODFs due to its excellent film-forming properties. However, its high cost restricts its sole use. To reduce costs, it is often blended with other polymers. Pure pullulan polysaccharide films are rather brittle, and plasticizers need to be added to improve their flexibility[23]. Qin et al. used different ratios of chitosan and pullulan sugar mixed with electrostatic spinning technology to prepare well-formed nanofibrous membranes. They successfully loaded aspirin onto the membranes, resulting in a membrane agent with good toughness[24]. Lavanya et al. successfully encapsulated the hydrophobic compound progesterone in Pullulan polysaccharide nanofibers using the electrospinning technique. The use of Pullulan polysaccharide as a raw material may promote better drug absorption and release[25].
Chitosan
-
Chitosan, also known as soluble chitin, is a polymer compound formed after deacetylation of chitin extracted from crustaceans. It is widely found in nature and has the characteristics of degradability, antimicrobial properties, and mold-forming properties[26]. The chemical structure of chitosan has many similarities with that of cellulose. The difference is that one of the hydroxyl groups on cellulose is replaced by an amino group. However, chitosan is insoluble in water and alkaline solutions, but it is soluble in weak acid solutions. After mixing carboxymethyl cellulose, sodium alginate, and chitosan in different ratios and using the casting method, well-formed membrane sheets were prepared with high flexibility. This indicates that chitosan can be used in the application of membrane agent-based products[27]. However, in current research, chitosan is often used as an adhesive for HPMC-based films to enhance drug permeability[28]. Few scholars have conducted research on the application of chitosan in ODF formulations.
Cellulose
-
Cellulose is a naturally occurring polymer material that is biodegradable, appearing as a white powder and easily soluble in water. It can be extracted from a wide range of sources and is readily available in nature. Cellulose is commonly derived from lignocellulose, which makes up 40% to 60% of the dry weight of plant cell walls and is the main component of plant cell walls[29]. Cellulose is a linear polymer consisting of β-1,4 glycosidic bonds, containing a large number of hydroxyl groups and hydrogen bonds. Natural cellulose itself is difficult to dissolve in common solvents. Usually, it is necessary to use some special solvent systems or carry out chemical modifications to improve its solubility[30]. This increases the process complexity and cost of preparing nanofiber membranes. Simultaneously cellulose can be modified to form different types of functional materials. In the field of pharmaceuticals, it is widely used for absorbing heavy metal contamination. It is also used in the preparation of cling film to slow down the aging and oxidation of fruits and vegetables. Additionally, it can be applied to the preparation of chemical materials, electronic metal plates, and other materials. It can also be applied to the preparation of chemical materials, electronic metal plates, and other materials. This process has a wide range of applications and is found abundantly in nature. It is a good raw material for film production[31].
Starch
-
Starch is a highly polymerized molecular compound, divided into straight-chain starch and branched-chain starch. A large amount of starch exists in many plants, such as potatoes, cassava, corn, and other plants. Starch has the advantages of being non-toxic, low cost, and naturally degradable. It is widely used in the fields of food, chemicals, biology, and materials. The semi-crystalline nature of natural starch leads to problems such as low solubility and poor mechanical properties. Generally, it needs to be modified chemically, enzymatically, or physically before it can be used in the preparation of ODFs[32,33]. Additionally, starch is an important carbohydrate that is an indispensable energy supply for the human body. Due to its special physicochemical properties, starch can be used in many processing methods[34]. Starch has an encapsulation effect, which contains microsphere organization that can play a protective role for substances, thus reducing the loss of substances in the human body. After the modification of porous starch and encapsulation of curcumin, the results show that modified starch microspheres play a good role in encapsulating curcumin[35]. Starch is hydrolyzed and oxidized to form a type of microgel, which increases the surface area and the number of pore spaces. Through electrostatic forces, the functional groups in anthocyanin can be attracted to each other, greatly improving the stability of anthocyanin[36]. Starch is widely used in food, not only for direct consumption but also for further processing and consumption when combined with other substances. The use of corn alkyd soluble proteins and modified starch, using electrostatic spinning technology, has successfully encapsulated vitamin B9 and prepared a well-formed membrane agent. This enhances the antioxidant activity of vitamin B9[37].
Pectin
-
Pectin, a natural polymer compound, is commonly found in the cell walls of plants and is part of a complex polysaccharide mixture. It can be extracted from fruits like passion fruit, citrus, and apple peel. Pectin is a plant-based polysaccharide made up of numerous glycans such as D-galacturonic acid, L-rhamnose, D-galactose, D-arabinose, arranged in a chain-like structure. Plants containing pectin are easily accessible and widely cultivated, and are now extensively utilized in food and biomedical applications. The addition of pectin to beverages enhances the viscosity of the beverage. The addition of pectin to jelly enhances the soft texture of the jelly. Pectin can also be used in the preparation of easy-to-swallow foods. Red grapefruit peel pectin, casein, and egg white protein were mixed as raw materials in different ratios. Good morphology edible films were prepared by the casting method, and the prepared films had high tensile strength and water vapor permeability[38]. However, only a few papers have used pectin as a film-forming agent in ODF formulations, which makes it difficult to compare studies and understand its true impact on film properties. In nature, there exists a large number of plant cell walls. Due to its natural mold-forming properties, pectin is widely used in the production of film products. Most pectin is non-toxic and moderately priced, making it a common raw material for the production of film agents[39].
In summary, the above materials are the main raw materials for the preparation of oral film agents. These materials are non-toxic and easy to obtain. Because of their stable performance, they are not easy to produce chemical reactions with other substances. They also have good film-forming performance, making them widely used in the preparation of film agents.
-
Development of an experimental design study for the development of an oral dissolution film for the administration of the antiepileptic drug topiramate with a pediatric focus. The main raw material of ODF is polysaccharide, but the vast majority of natural polysaccharides, due to their chemical properties, will carry some special odor or taste. After dissolution, its intermolecular forces will change, and its physicochemical properties will also change. Therefore, in the production of oral membrane agents, to improve viscosity and eliminate undesirable flavors (Table 2), appropriate amounts of cross-linking agents and sweeteners should be added[40]. Commonly used cross-linking agents include glycerol and polysorbate 80. Commonly used sweeteners include sucralose, aspartame, and sugar. Citric acid can be used as a corrective flavor[41]. Because the oral membrane agent is easy to carry and has a small specification, there are certain limitations in its contact area with the oral cavity. This is because the oral membrane agent needs to be disintegrated in the oral cavity to release the substances in the membrane. However, some film-forming materials are not easily dissolved in the oral cavity, resulting in incomplete release of the encapsulated substances or a prolonged release time. Therefore, it is necessary to add a disintegrant to promote the release of the membrane agent in the oral cavity and to improve the release rate of the encapsulated substance. Commonly used disintegrants include cross-linked sodium carboxymethyl cellulose, sodium hydroxyacetic acid starch, and cross-polyvinyl ketone[42]. A fast oral fast-dissolving film containing downy hooker bark extract was prepared by adding cross-linked sodium carboxymethyl cellulose to it, using Pullulan sugar as the raw material. The resulting membrane agent also proved to have a short onset modulation of plasma levels within 30 min in drug release[43].
The role of plasticizers
-
Plasticizer, also known as a crosslinking agent, plays a role in increasing the intermolecular forces in a solution. When polysaccharide solutions are processed using different methods to create a membrane agent, wrinkles, or fragility in the texture may occur. To form a tight and dense film, a crosslinking agent needs to be added to the solution to improve the membrane's flexibility and reduce its brittleness. This results in a better membrane morphology. The commonly used plasticizers include propylene glycol, polyethylene glycol, and glycerol, with glycerol being the most commonly used[44]. Glycerol has a moisturizing and plasticizing effect, which can prevent the film agent from breaking due to drying or other factors. As the film agent is stored for a longer time, the water in it will evaporate, causing the film agent to become wrinkled. Therefore, glycerol can be added during the production of the film agent to prevent drying and wrinkling. Additionally, the addition of glycerol can increase the thickness, elongation at break, and tensile strength of the film agent[45]. Glycerol is a colorless and odorless substance. Glycerin is a colorless and odorless viscous liquid, which is widely used in the food chemical industry and other fields. Glycerin is widely used because of its low price, suitable storage conditions, and lack of excessive requirements. A certain amount of cross-linking agent was slowly added to the solution in appropriate amounts during mixing, and the resulting film had good toughness and a more gentle shape[46]. Soluble soybean polysaccharide was used as the membrane matrix, and varying amounts of glycerol were added to observe the different properties of the membrane. The results showed that when 20% glycerol was added, the membrane reached optimal values for gas barrier, heat resistance, and tear resistance[47].
Colorants
-
The original color of the substance will change when it is subjected to changes in the external environment, such as heating, which will cause the color to become lighter. The color will also change under different pH conditions, so the color of the membrane agent needs to be improved. Colorants are divided into two categories: natural colorants and synthetic colorants. Natural colorants, as their name suggests, are extracted from natural substances, such as carrots, which contain large amounts of natural carotenoids. Anthocyanins from blueberries or purple kale, cochineal, are the most commonly used animal pigment, and erythrina is a microbial pigment[48]. Synthetic pigments are classified as azo and non-azo pigments according to their different structures. The main way to differentiate them is whether they contain an even number of nitrogen atoms or not[49].
Flavoring agents
-
Since some substances have naturally bad odors, it is necessary to add food additives to the oral film to improve the original bad odor and taste. Oral films sometimes need to add sweeteners, such as white sugar, sucralose, xylitol, and other substances. Citric acid is widely used in all kinds of food and many beverages. Food-grade citric acid is often added to food. Adding citric acid to the thin film agent can give it a refreshing taste, and the preservative effect of citric acid can extend the preservation time of the thin film agent to a certain extent[50]. Therefore, flavoring agents need to be added to oral membranes to improve the flavor of the membrane agent.
Table 2. Film forming auxiliary materials and their functions.
Purpose Frequent species Effect Plasticizer Glycerin, polyethylene glycol, propylene glycol Enhanced intermolecular crosslinking[51] Colorant Natural pigments, synthetic pigments Protect the substance[52] Condiment Citric acid, sucralose, xylitol The substance is protected to improve the bad odor carried by the substance itself[53] -
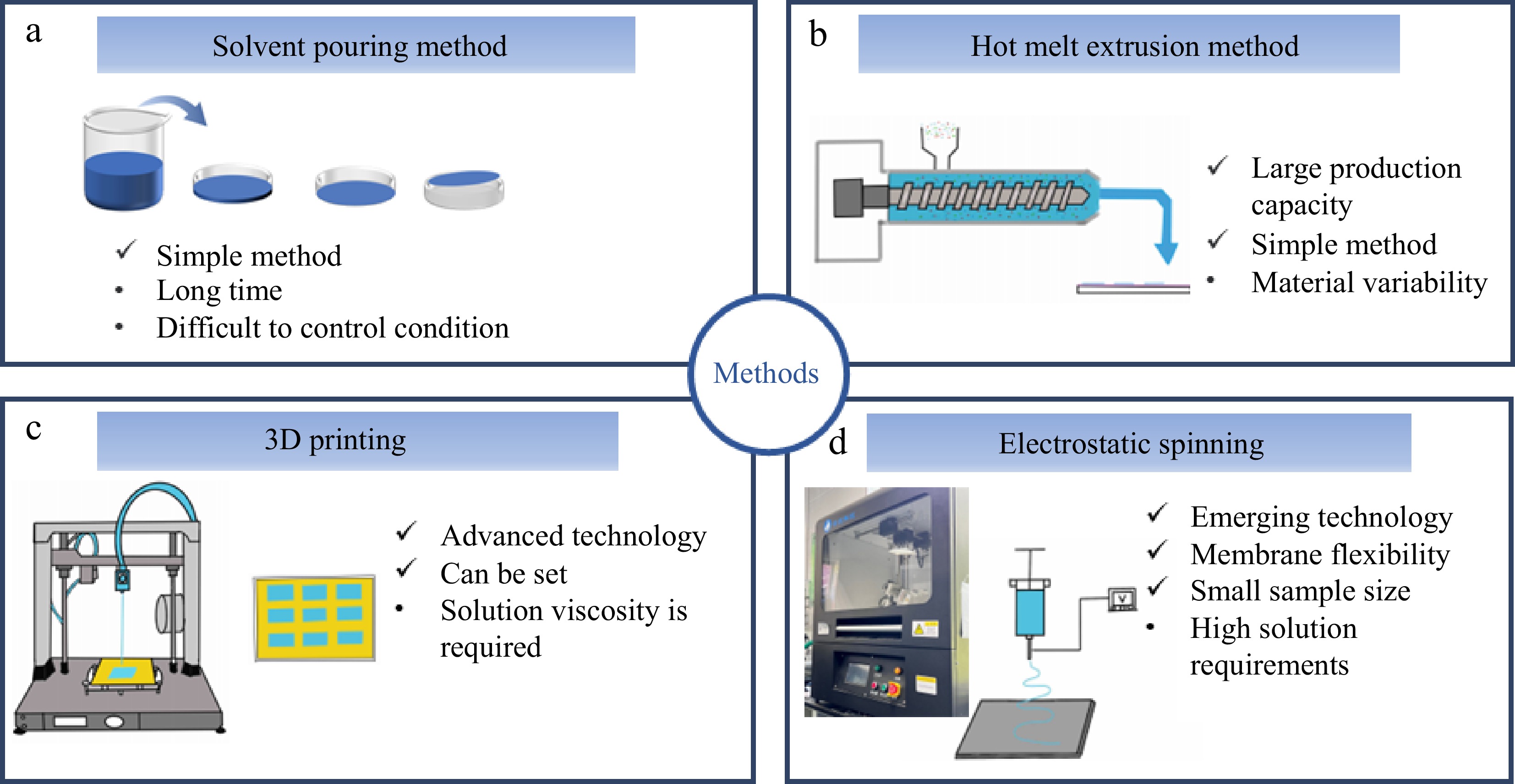
The most common preparation methods for ODF are casting and hot melt extrusion. With the gradual development of industrial technology, various large-scale intelligent machinery is being integrated into the food industry, and the potential for new developments in the combination of food and machinery is being realized. Examples of this include 3D printing technology and electrospinning technology[54].
Calendering
-
The casting process is one of the most common and commonly used methods for manufacturing film agents, mixing components, and then heating or mixing at room temperature (Fig. 3a). In this process, the film-forming material and encapsulated substance are completely dissolved and placed in a group of carriers such as a tank, glass plate, or tetrafluoroethylene plate. The mixture is then left to stand for a period of time in a high-temperature oven, or at room temperature. During this time, it can be gently shaken to remove any bubbles in the liquid. After a period of time, the film is unveiled[55]. The fluidized method is easy to operate. The solution is prepared and dried for a period of time to obtain the film agent. When fluidizing, one should pay attention to the drying temperature. If the temperature is too high, it will cause wrinkles in the film agent. If the temperature is too low, it will prevent the liquid from solidifying, resulting in an incomplete film formation[56]. However, this method takes a long time and has greater limitations, which is more restrictive for substances with low viscosity and poor film formation, and is prone to wrinkles when drying. The thickness of the film is not controllable. Some substances can be dried at room temperature to form a film, but others need to be dried at high temperatures. Some substances are easily denatured at high temperatures, so the temperature must be strictly controlled. For heat-sensitive substances, low-temperature drying should be used. Increasing the temperature results in a reduction of the required drying time. However, a higher temperature causes the film to have lower strength. This lower strength makes the film more likely to break and impairs its tensile properties. Therefore, different drying temperatures and times should be selected according to the different properties of the substances. A nanocellulose/chitosan/poly (vinyl alcohol) composite membrane biofilm was prepared using the casting method, and the composite membrane was successfully obtained by drying it at 60 °C for 2–3 h. The resulting biofilm was complete and had good properties[57].
Hot-melt extrusion method
-
Hot-melt extrusion, also known as solid phase dispersion extrusion, involves heating the encapsulated substance, film-forming material, and other auxiliary materials at high temperatures until they are completely melted into a uniform dissolved mixture (Fig. 3b). This mixture is then melted at a fixed speed using a single screw or twin screw, and is pulled out and formed through a hole on the abrasive tool to create the required film. The film is dried until it takes its final shape. According to the desired particle size and shape, the film is dried to the desired size and shape. After that, it is cut to the desired size[57]. The hot-melt extrusion method is highly adaptable for large-scale manufacturing. Substances that are stable at ambient temperature but decompose when heated are particularly amenable to this approach. In contrast to methods that rely on solvents for dissolution, the hot-melt extrusion method achieves the transformation of the substance into a molten state primarily through high-temperature heating. The method does not require additional heating, which can save time. Additionally, some substances can be more uniformly mixed with auxiliary materials under the heating conditions. The process requires precise control of temperature, speed, feed volume, feed time, and pressure. The hot melt extruder can precisely control the required temperature and extrusion speed, making it convenient for large-scale industrial production[58]. The hot melt extrusion method does not require overly complex operations and is suitable for water-sensitive substances, making it a cost-effective option. Hydroxypropyl cellulose and chitosan were used as the main raw materials, mixed with xanthan root, and then tableted at 150 °C. This process improved the preservation time of xanthan root, the active substance[59]. Kanaujia et al. prepared an oral dissolution film containing sirolimus using the hot melt extrusion method. The film was able to significantly improve the drug's dissolution profile and remained stable for 3 months with good performance. This effectively enhanced the utilization of sirolimus in oral delivery[60].
3D printing technology
-
Also referred to as additive manufacturing or rapid prototyping technology, 3D printing technology has seen significant advancements in recent years due to the integration of computer, optical, mechanical, and chemical disciplines. By combining artificial intelligence with computer-aided design (CAD) software control, this technology allows for the creation of three-dimensional objects by layering materials (Fig. 3c). It is widely utilized in various industries such as materials, healthcare, and food[61]. Procusini is a German company that focuses on providing food for the restaurant, dessert, and confectionery industries. The company can print different flavors in various styles. The core principle of this technology is to create three-dimensional patterns by manufacturing layers, which are then superimposed on each other. 3D printing is used in all major fields due to its simplicity, affordability, and technological advancement. 3D printers are connected to a computer through a monitor and then print according to pre-set specifications and shapes[62]. 3D printers are classified into two types: fusion printers, which are used for heated substances, and electro-fluid 3D printers, which are used for more viscous substances[63]. Fusion 3D printing is divided into four parts: the engine, gear module, heated extrusion head, and printing substrate. This means that the substance is heated at a high temperature and then dissolved. The molten substance is extruded from the nozzle and stacked according to the pre-designed pattern, resulting in a final product after all layers are stacked. 3D printing is different from previous methods in that it combines network technology, making it easier to control. The film-making process can be carried out by setting various parameters. This sets 3D printing apart from previous methods, as it combines network technology and is easier to control. The film-making process can be carried out by setting various parameters.
Elbadawi et al. conducted research using 3D printing technology with Pullulan polysaccharide and hydroxypropyl methylcellulose as raw materials and caffeine as the loaded model drug. The experimental results indicated that the spatial deposition of the membrane can be regulated by means of 3D printing technology. Additionally, during the research process, it was observed that the properties of Pullulan polysaccharide can influence the mechanical strength of the hybrid membrane, which provides directions for future research[63].
Electrostatic spinning technology
-
Electrostatic spinning technology is a novel technology that was first proposed in 1934. The principle is to use electrostatic force to transform polymers into nanometer ultrafine fibers (Fig. 3d). When the electric field force is large enough, the polymer droplets overcome surface tension to form a jet of fine streams. This results in the formation of a stable Taylor's cone, continuously ejecting well-formed fibrous filaments. Layer by layer, these filaments form a dense texture of fibrous mat. This technique does not require a large amount of solution, and the membrane agent prepared is a nanofiber membrane, which is more easily absorbed[64]. Uniaxial electrostatic spinning requires a single injection needle, which can directly eject well-formed filaments when the power is turned on. Coaxial electrostatic spinning requires two needles. This method can form a shell-nucleus structure, which has a good protective effect on the substances in the nucleus. The two syringes are loaded with shell liquid and nucleus liquid. Then, a coaxial needle connects the two syringes, and the shell liquid wraps the nucleus liquid to form a shell-nucleus structure. The electrostatic spinning machine is mainly composed of four parts: a propulsion pump, a syringe, a high-voltage power supply, and a receiver. The positive pole is connected to the syringe, and the receiver is connected to the negative pole. After the switch is turned on, the solution is sprayed out from the syringe under the action of the electric field force and is received on the receiver plate[65]. The receiver can be either a constantly rotating roller or a receiving steel plate. During the electrostatic spinning process, it is essential to monitor multiple parameters: the solution viscosity, pushing speed, voltage, temperature, humidity, rotating speed of the receiving drum, and the distance between the receiving plate and the receiver. It is necessary to observe that the fiber filaments spun by the electrostatic spinning machine are in the shape of a Taylor cone.
Ravasi et al. successfully encapsulated sildenafil in nanofibers by electrospinning using pullulan polysaccharide as the raw material. The prepared orally soluble film of sildenafil can be immediately dissolved in saliva without the need for water[66]. Hirsch et al. also achieved successful encapsulation of probiotic bacteria in nanofibers through electrospinning, with a bacterial survival rate of over 80%. The high cell number can be preserved for up to one year[67]. The nanofiber mats produced using the electrospinning method have a compact texture and good morphology. However, the nanofiber filaments are very fine, resulting in a long spinning time and high solution requirements. This method is now widely used in biomaterials such as gingival patches and wound aids. The membrane produced by electrospinning is compact and the nanofibers are better absorbed than particles and macromolecules, making it highly promising for various applications[68].
-
There is a significant difference between oral fast-dissolving films prepared using various raw materials and preparation methods. The resulting film must be evaluated for its mechanical properties and subsequent absorption ability in the oral cavity. The successfully prepared films should have an appropriate thickness, strong folding resistance, and a suitable disintegration time. It is essential to consider that films made from different raw materials may require different storage conditions, so their water content and surface pH should be measured[69].
Mechanical properties
-
Due to the extremely thin nature of the nanofiber membrane, to ensure the accuracy of the measured data, a micrometer screw gauge is usually used to measure its thickness at five different positions. Specifically, the membrane will be measured five times at each position, and the average value will be calculated. This average value will be used to observe its texture, grain cracks, flexibility, and uniformity.
To ensure that the membrane agent is easy to carry and has good physical properties, the folding resistance is typically used to assess its mechanical properties. The membrane is folded repeatedly at the same point until it breaks, and the number of folds is noted. Generally, a higher folding resistance indicates greater mechanical strength of the membrane and a lower likelihood of breakage[70]. Tensile strength is calculated by dividing the force applied when the membrane breaks by the cross-sectional area of the strip, and is expressed as force per unit area.
$\rm Tensile\; strength\; (TS)\; (MPa) = F/(L \times d)$ where, TS = tensile strength; F = maximum tension of the sample at breakage; L = Film width; d = Thickness of film.
Because oral films need to be melted in the mouth, there are significant differences in the disintegration time in the oral cavity due to the different molecular weights of sugar and varying ratios of film-forming raw materials. A film with a maximum disintegration time of less than 60 s is known as a fast-dissolving orally soluble film. The disintegration time is recorded by simulating the human body's oral saliva and visually inspecting the film in 25 mL of phosphate buffer solution at pH 6.8, at a temperature of 37 ± 0.5 °C. The in vitro disintegration time of a film with an area of 2 × 3 cm2 is recorded, and the time at which the film starts to rupture is considered the disintegration time.
Water content of the film
-
Before packaging, it is important to determine the moisture content of the film to prevent film corruption during the preservation process. The moisture content of the film must be within a certain range to avoid issues such as stickiness or crumpling. Polysaccharides are commonly used in film-forming materials, and high moisture content can cause the film to become sticky and change in morphology, while low moisture content can lead to crumpling and changes in morphology during preservation. Therefore, it is necessary to measure the moisture content of the film to ensure proper packaging conditions. The moisture content of the film can be measured by weighing the film, placing it in a desiccator with calcium chloride for 3 d, and then reweighing it to calculate the moisture content using a specific formula.
${\text{%}}\rm \; Moisture\; content =\dfrac{Initial\; weight \;final\; weight}{Initial\; weight}\times 100$ The pH of the diaphragm varies greatly between different materials and substances encapsulated within it. Some substances are easily affected by acidic or alkaline conditions, so the preservation method should be chosen based on the diaphragm's pH. Therefore, it is important to measure the pH of the diaphragm. To do so, the diaphragm was dissolved in a beaker containing 5 mL of distilled water and stirred until completely melted. The pH was then measured using a pH meter[71].
Encapsulation rate and oral absorption
-
The most important point in evaluating the success of an oral film agent is the rate of substance encapsulation. Usually, the higher the rate of substance encapsulation, the greater the protection of the encapsulated substance is proven. Al-Mogherah et al. prepared a fast-dissolving membrane of venlafaxine hydrochloride for oral use. The oral dissolution of the membrane was dissolved in a phosphate buffer solution, and the absorbance was measured using a spectrophotometer as a means of evaluating the acceptability of the membrane to encapsulate the content of venlafaxine hydrochloride[72]. The total phenol content of the membrane was measured by dissolving the membrane sample in 50% ethanol and measuring its absorbance using a spectrophotometer[73]. Alternatively, human oral saliva was used to simulate substance dissolution with the temperature set at 37 ± 0.5 °C. The composition of the simulated saliva consisted of 12 mM potassium dihydrogen phosphate, 40 mM sodium chloride, and 1.5 mM calcium chloride, and the pH was adjusted to 6.8 using sodium hydroxide. The dissolved substances were measured using a high-performance liquid chromatograph.
Oral mucosal adhesion
-
The site of action of the orally soluble membrane is within the oral cavity, and mucosal adhesion is considered to be the strength required to separate the ODF from the model tissue. To ensure that the prepared membrane agent can be successfully adhered to the oral cavity, the oral adhesion of the membrane agent needs to be determined. The adhesion of the patch was measured using a TA.XT2 plus mass spectrometer (Stable Microsystems SMD, England) as a measuring instrument. The contents of the chicken pouch, as well as tissues such as the surface fat, were removed. The membrane patch was then applied to the surface of the inverted chicken pouch, and the adhesion work and peak separation force were used to determine the bioadhesion strength of the membrane patch[74]. Rabbit buccal mucosa has a similar structural organization to human oral mucosa, so it is widely used in experiments on the transport system of substances in the oral cavity. Fresh rabbit mucosa can be used for adhesion experiments, as well as permeability experiments, after removing excess tissue[75]. Adhesion strength was determined using a texture analyzer, with rabbit buccal mucosa as the substrate. Briefly, the buccal mucosa was fixed to a fixation table, while a membrane with the appropriate dimensions was attached to the analyzer's probe. The tissue membrane was moistened with simulated saliva, and the removable probe was gradually slid downward until it came into contact with the mucosa. It was then held for 1 min to determine film adhesion (Stable Micro Systems, Surrey, UK).
Successfully prepared oral films should be uniform in form, without wrinkles, and uniform in color as determined by the naked eye. The thickness of the film varies according to its intended use, but it should be consistent throughout. The bioavailability of orally dissolving films of fexofenadine hydrochloride, prepared using the solvent casting method, is 4.15 times higher than that of tablets. The thickness of the film ranges from 0.15–0.35 mm, and its weight falls between 175.6–208.5 mg[76]. However, due to the different substances and encapsulated raw materials, there may be variations in the dissolution time. For instance, curcumin encapsulated with pvpk90 showed complete release within 30 s, and its folding durability was greater than 300, indicating good resistance to folding. Since oral soluble films come into direct contact with the oral cavity, the pH of the prepared agent should not cause excessive irritation. The pH range of the prepared fast-dissolving oral film tablets was 5.8 ± 0.08–6.3 ± 0.26, which indicates that they do not irritate the oral mucosa.
Evaluation of oral dissolving film absorption
-
The main absorption site of ODF is in the oral cavity. Very few substances will enter the esophagus and gastrointestinal tract with saliva, and the vast majority of absorption occurs in the oral cavity. Therefore, oral absorption is evaluated using frequently used methods such as oral cellular absorption evaluation and animal models. TR146 cells belong to the human oral buccal carcinoma cell line, also known as human esophageal squamous carcinoma cells. They are a commonly used cellular model for determining the absorption of substances in the oral cavity[77]. Castro et al. used guar gum and alginate as film-forming materials, and caffeine as the encapsulated substance, to prepare buccal membranes using the casting method. The buccal fast-dissolving membranes produced using TR146 cells were determined not to impair cell viability, and caffeine could be adsorbed by the cells[78]. Male rabbits, which were kept for a period of time, were divided into different experimental groups. One group was anesthetized, and the prepared oral film agent of rizatriptan was applied to the inner wall of the mouth. The other group was given an equal dose of liquid rizatriptan solution as a control group. After a period of time, the concentration of the drug in the bloodstream was measured[79]. The pramipexole dihydrochloride containing pramipexole dihydrochloride, which was prepared by Pamlényi et al. for the treatment of Parkinson's disease, contained sodium alginate (1% w/w), hydroxypropylmethyl (2% w/w), glycerol (1% w/w), and pramipexole dihydrochloride (0.0793% w/w). The optimal ratio for application of the buccal mucous membrane tablet was found to be sodium alginate (1.5% w/w), hydroxypropylmethyl (1.5% w/w), glycerol (1% w/w), and pramipexole dihydrochloride (0.0793% w/w) cellulose, which can be solubilized in 5 min[80].
-
ODF has promising applications in the food and pharmaceutical industries. Traditional tablets and granules often require a large amount of water for consumption, making them inconvenient to carry around. Liquid dosage forms can also pose a choking hazard during administration. ODF offers a solution by encapsulating substances in a thin film that can be easily absorbed through the oral cavity, reducing the loss of active molecules due to the first-pass effect. This delivery method is particularly beneficial for individuals with swallowing difficulties, such as the elderly and children. ODF quickly disintegrates in the mouth, allowing for rapid release of the encapsulated substance. This is advantageous for substances that are not stable in the intestines and may undergo significant loss when passing through the liver. By bypassing these barriers, ODF ensures efficient absorption of the active molecules. Additionally, ODF offers advantages such as precise dosage control, compact size, ease of transportation, and no need for water during administration. The film can also provide further protection for the encapsulated molecules. Overall, ODF presents a convenient and effective way to deliver active molecules directly into the bloodstream, improving bioavailability and enhancing patient compliance[81].
In recent years, there has been a gradual increase in the variety of film-forming raw materials due to the development of chemical and mechanical industries. This has led to advancements in film-making methods. Oral instant film tablets are now being used in some clinical drugs and have gained popularity in foreign countries for treating post-chemotherapy vomiting, oral infections, hypertension, schizophrenia, and Alzheimer's disease[82]. However, there is a limited variety of oral soluble films in China, and research on oral soluble films in food is lacking. The portability, universality, and effectiveness in reducing the intestinal first-pass effect of oral fast dissolving film tablets make them significant. Therefore, the use of oral fast dissolving film agents as a new delivery method in the food industry is expected to be a key area of research in the future.
This work was supported by the National Natural Science Foundation of China (32202074), and the Scientific Research Foundation of Shenyang Agricultural University (880418027).
-
The authors confirm their contributions to the paper as follows: writing-original draft: Zhao M; visualization: Sun F, Bao Y, Li J; investigation: Ao G, Li Y; supervision: Shu C, Si X, Wang Y, Tan H; project administration: Li B, Zhou Y, Yang B, Makarov SS, Chudetsky AI; conceptualization: Tian J. All authors reviewed the results and approved the final version of the manuscript.
-
All data that support the findings of this study are available from the corresponding author upon reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao M, Sun F, Bao Y, Li J, Ao G, et al. 2025. Research progress and overview of oral instant diaphragm tablets in food field. Food Innovation and Advances 4(4): 490−499 doi: 10.48130/fia-0025-0047
Research progress and overview of oral instant diaphragm tablets in food field
- Received: 25 December 2024
- Revised: 13 April 2025
- Accepted: 15 April 2025
- Published online: 28 November 2025
Abstract: Oral Dissolving Film (ODF), a new type of oral drug delivery formulation, offers advantages such as low weight, small size, convenient portability, water-free administration, and rapid dissolution in the oral cavity. It can effectively bypass the first-pass effect of the liver and enhance the bioavailability of substances. This article delves into the applications of ODF in the food field. It elaborates on the film-forming raw materials, including polysaccharides and their derivatives, non-toxic polymers, etc., and the functions of various auxiliary materials. Additionally, it introduces multiple preparation processes such as the casting method, hot-melt extrusion method, 3D printing technology, and electrostatic spinning technology, along with their advantages and disadvantages. Moreover, the measurement and evaluation indicators of ODF, such as mechanical properties, disintegration time, moisture content, pH value, encapsulation rate, oral absorption, and mucosal adhesion, are explained. Currently, ODF has been applied to foreign clinical drugs, yet there is limited research and application in the food field in China. Given its numerous advantages, ODF holds great potential for future research and application in the food industry.